What types of risks are covered by Risk management?
It covers the harms related to patients, users or/and environment.
What is the difference between Design controls and Risk management?
Design Controls prove that the Medical Device is safe, effective and meets the indications for use, and help reduce product risks. In parallel, Risk Management intends to identify, evaluate, analyze, assess and mitigate potential product issues. Indeed, there is a correlation between these two notions, however, the perspectives are different.
What are the steps of the Risk Management process?
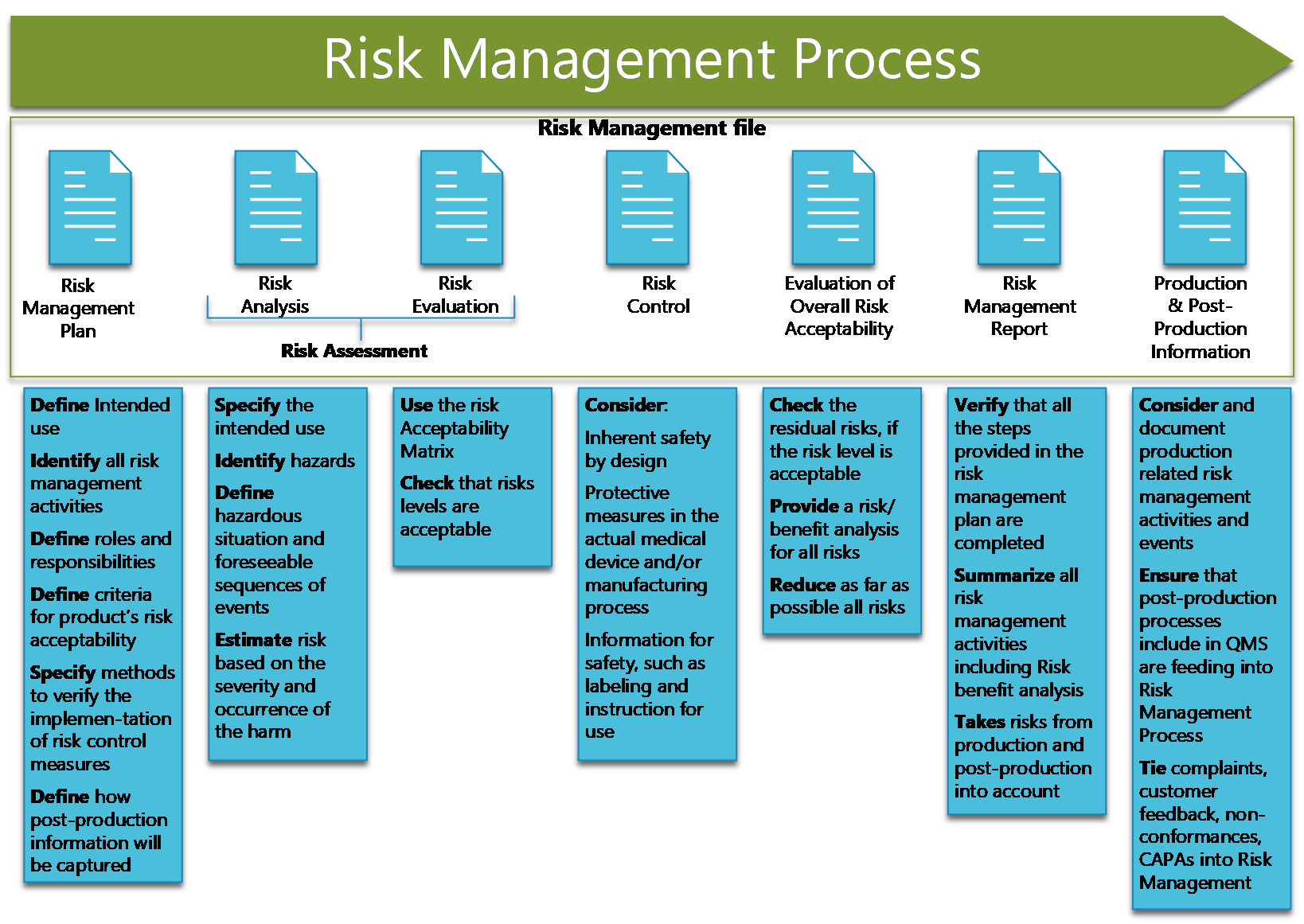
A risk Management file must include:
- Risk management Plan
- Risk Analysis
- Risk Evaluation
- Risk control
- Evaluation of Overall risk acceptability
- Risk Management Report
- Production and Post-Production Information
These fields define the steps of the Risk Management process.
Author: Alix Auter, Life Science Consultant KVALITO
KVALITO is a strategic partner and global quality and compliance services and network for regulated industries. To learn more about our service please visit us on www.kvalito.ch
If you would like to benefit from KVALITO’s expert services, feel free to send us an email to contact@kvalito.ch.
Are you looking for an exciting and challenging position as a consultant in Europe? Send us your complete application to recruiting@kvalito.ch.





