What kind of sterilization methods can be used for a medical device?
There are different methods for the sterilization of medical devices, using gas and steam or radiation.
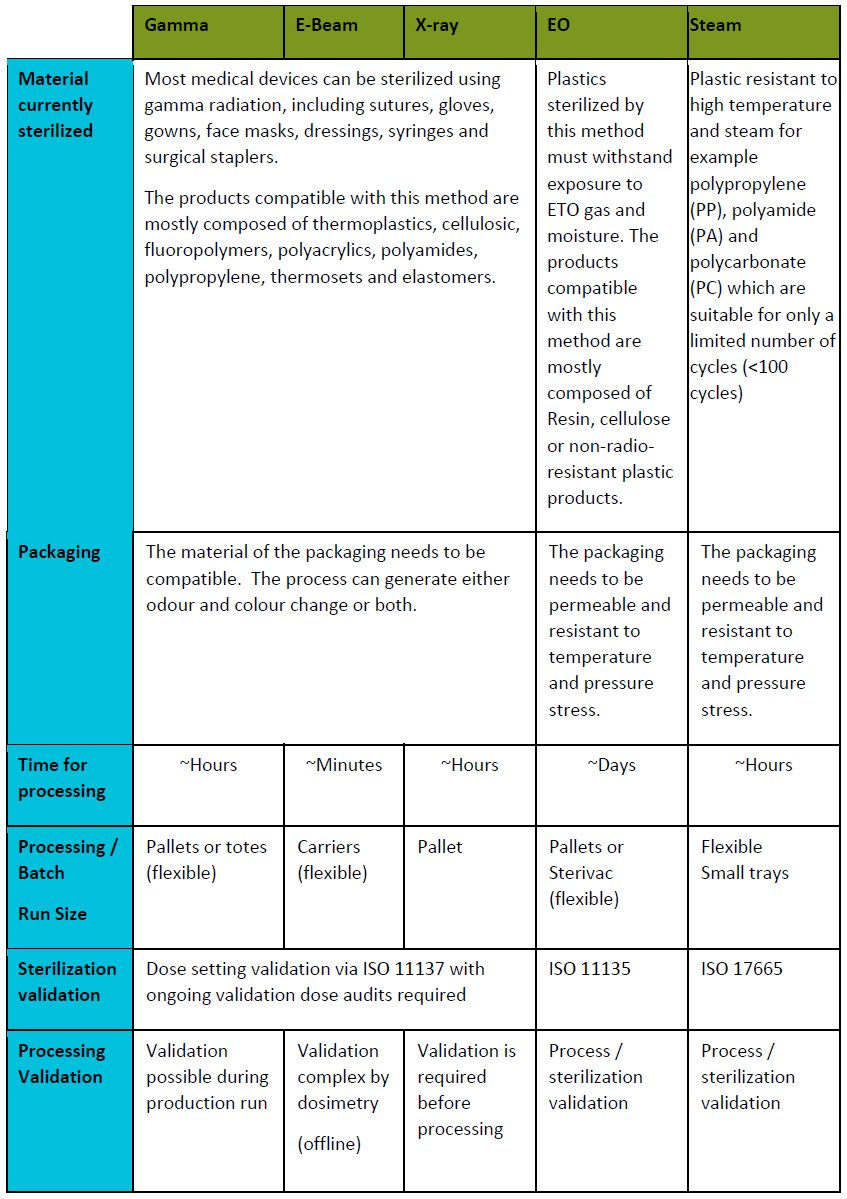
1. Radiation methods:
The principle of this form of sterilization treatment is to bombard the sample with high energy electrons or high energy electromagnetic radiation. This activity leads to the formation of unstable free radicals, molecular ions, and secondary electrons. These products react with molecules and fracture their chemical bonds. DNA and RNA are sensitives to this sterilization approach.
The advantage of this technique is that products can be sterilized through radiation after packaging due to the penetrating power of the incident gamma-ray.
Nevertheless, some drugs like heparin cannot be irradiated because there is a risk of changing the composition of the molecule, making it ineffective or dangerous for human health.
Sterilization by ionizing radiation using:
- Gamma: Cobalt 59 is converted in cobalt 60 by neutron bombardment. The product moves around the energy source and becomes sterilized. A water pool is provided in the gamma plant to protect people against radiation.
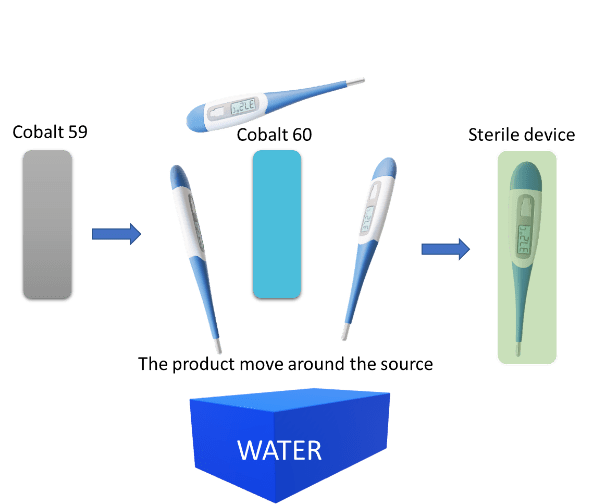
Figure 1: Gamma irradiation sterilization process. Copyright 2020, KVALITO Consulting Group
Electron beam is also known as beta radiation: Electrons are accelerated through an evacuated tube and focused into a beam. The beam scans the product. This technique has a limited depth of penetration for a high dose, the opposite of gamma radiation.
X-ray: An electron-to-photon conversion is enhanced by interposing a metal target between the electron beam and the product.
2. Gas and steam sterilization technologies:
Ethylene oxide: Alteration of the microbiological contaminant DNA by a chemical reaction. Ethylene oxide replaces the hydrogen atoms on the DNA strand, which conduct to the stop of molecule normal life-supporting functions and death of microbial agents. The only real constraint is that the packaging must be permeable to ethylene oxide.
- Moist heat: variation of the sterilization cycle. The air in the autoclave is heated, and the pressure is lowered so that the air drops.
How do we validate a medical device sterilization process?
For all techniques, an Installation Qualification (IQ), an Operational Qualification (OQ) and a Performance Qualification (PQ) must be created, executed and documented.
Sterilization by radiation requires one more step during the Performance Qualification process than gas and steam sterilization methods. Indeed, for such radiating sterilization methods, the irradiation dose must be verified with dosimeters placed throughout the load to assess minimum and maximum dose points.
Nonetheless, a microbiological validation is needed for all the sterilization methods to ensure and validate the sterility of products.
Author: Alix Auter, Life Science Consultant KVALITO.
KVALITO is a strategic partner and global quality and compliance services and network for regulated industries. To learn more about our service, please visit us on www.kvalito.ch
If you would like to benefit from KVALITO’s expert services, feel free to send us an email at contact@kvalito.ch.
Are you looking for an exciting and challenging position as a consultant? We look forward to receiving your complete application at recruiting@kvalito.ch.





