A continued trend in novelty saw the FDA (Food and Drug Administration) approving many structurally innovative drugs in 2020.
Powerful innovation of new drugs, biological products, and medical care usher in the promise of new and better treatment options for improved health outcomes for patients. Deeper insights into biology continue to proliferate, accompanied by faster identification of novel targets and their associated expressions.
An inquiry into innovation in the pharmaceutical industry would be incomplete without a brief look at the history of drug discovery and the events that led to the introduction and development of new therapeutic agents.
The earliest medicinal products were made from plants, roots, herbs, vines, and fungi. Until the mid-nineteenth century, it was nature’s pharmaceutical toolbox that could treat disease (and continues to do so in traditional medicine). Early pharmaceutical companies were spin-offs from the textile and synthetic dye industries, which provided a plentiful supply of organic chemicals.
The modern pharmaceutical industry traces its origins to two sources: apothecaries that moved into wholesale production of medicines such as morphine, quinine, and strychnine in the middle of the 19th century and dye and chemical companies that established research labs and discovered medical applications for their products starting in the 1880s. As a sedative-hypnotic agent, chloral hydrate was introduced in 1869 as the first synthetic drug. As byproducts of coal-tar production, aniline and p-nitrophenol were the first analgesics and antipyretics. The extract of white willow bark has been used to treat fevers and inflammation for centuries. Salicylic acid’s active property in white willow had a bitter taste and irritated the stomach membrane, but a simple chemical modification improved efficiency and taste. [1] Acetylsalicylic acid, more commonly known as Aspirin ®, was invented by Bayer in 1899 and was the first blockbuster drug. Aspirin is a small molecule drug, measuring only 180 Da (Daltons). Small molecules have a molecular weight of < 900 Da and comprise up to 90% of pharmaceutical drugs.
Small molecule therapeutic agents targeting proteins have been the mainstay of the pharmaceutical industry. Their success and their broad scope of therapeutic application, for example, in organ transplantation, as probes in chemical biology, as diagnostic agents or as facilitators of cell and gene therapy, is partly due to their inherent properties, including their ability to cross biological barriers and modulate multiple biological targets. The modular structure of small molecules and their high availability for chemical synthesis is an important characteristic, enabling the rapid alteration of the chemical structure and systematic improvement of their properties.
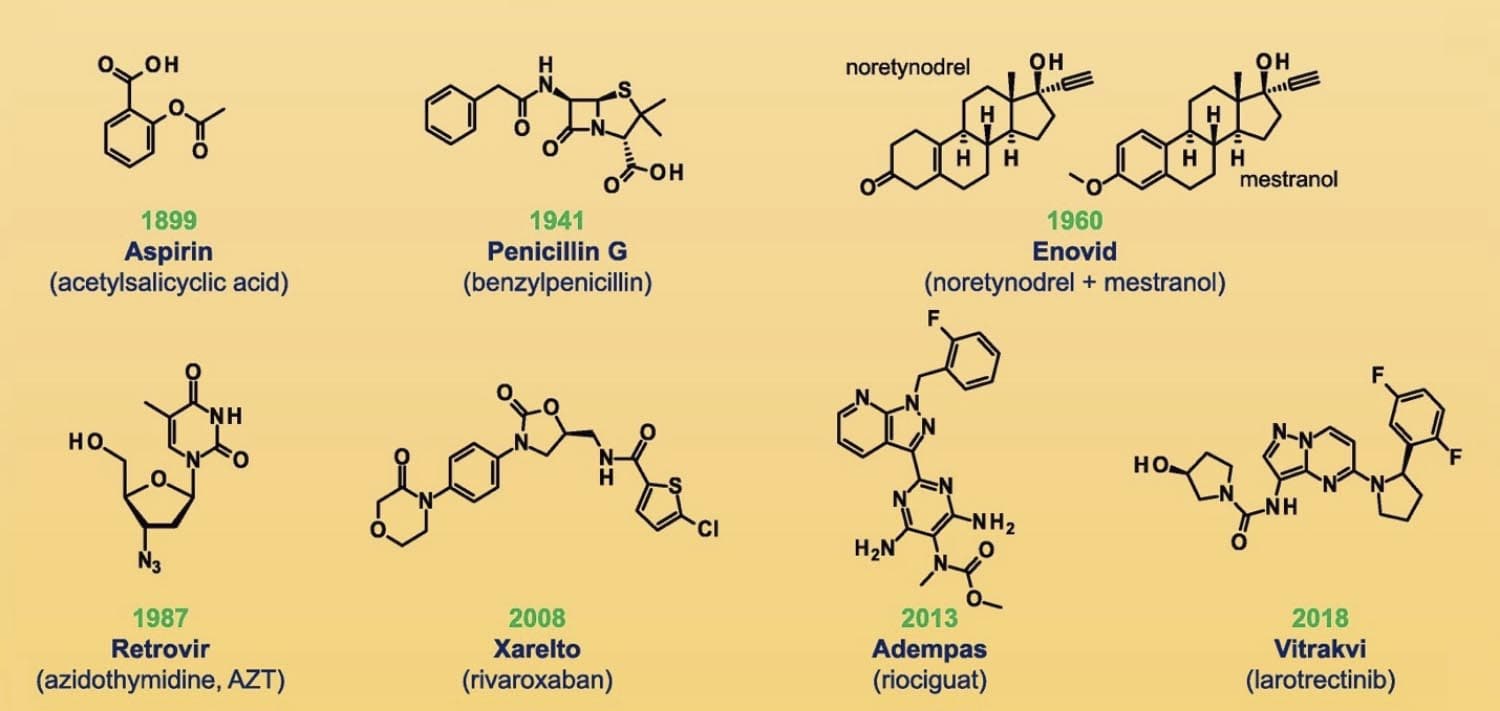
Figure 1 Chemical structures of selected small-molecule drugs [2]
FDA Approvals in 2020
Small molecules accounted for 90% of all approved medicines and around 75% of all new medicines approved in the United States in 2020.
Notwithstanding the limitations presented by COVID-19, the FDA approved 53 new therapeutic medications in 2020. This count is the second highest on record, equal to the number of FDA-approved medications in 1996, and not far behind 2018’s notable count of 59.
In a publication, Nogueira and de Souza (2020) identified that small molecules continued to play a central role in innovative treatments, representing around 75% of all drugs accepted by FDA.” [3]
The 53 FDA-approved drugs in 2020 included 40 small molecules (36 new chemical entities and four new diagnostic agents) and 14 biologic drugs. The drug categories included peptides, antibodies, antibody-drug conjugates, small molecules, and oligonucleotides.[4]
20 of these small-molecule drugs were structurally novel pioneers, comprised of at least one new molecular entity (NME) derived from a new molecular shape. 31 were cyclic small-molecule drugs (not counting diagnostic imaging agents), representing the principal drug category. 2020 was marked by the industry’s increased focus on cancer, with the FDA approving 18 oncology products.
Molecular Shape
To design a safe, effective, and commercially successful small molecule drug, a target must have the ability to be therapeutically modulated by medicines, and the molecule must have the right attributes to access that target (lock and key). It is interesting to consider the relationship between the shape, structure, innovativeness, and clinical and commercial success of small molecules. Indeed, medical products with a structurally innovative shape are twice as likely to become blockbusters, surpassing the benchmark of $1 billion in sales. They also have a 2.5 times higher chance of becoming breakthrough therapies (offering a superior standard of care than existing therapies).
How Can We Measure Innovation in Pharma?
Innovation in the pharmaceutical industry is challenging to measure. Various methods have been applied to assess the level of innovation in pharmaceutical products; however, many come with limitations.
- New molecular entities (NMEs): The number of NMEs approved by the FDA has been used to measure innovation. However, this measures numbers and not individual innovativeness; the level of innovation in one NME is not equal to that of the next.
- Breakthrough Therapies: The FDA stipulates that the criteria for breakthrough therapy designation require preliminary clinical evidence that demonstrates the drug may have substantial improvement on at least one clinically significant endpoint over available therapy. [5] Drug candidates receive priority review from the FDA when preliminary clinical trials indicate that they may provide substantial treatment advantages over existing options for patients with severe or life-threatening diseases.
- First-in-class medication A new and unique mechanism of action that treats a particular medical condition.[6]
- Orphan Drugs (therapeutic need): For patients with rare diseases, the approval of so-called ‘orphan’ drugs can provide new hope for improved quality of life and, in some cases, survival. This set of drugs and first-in-class drugs have been described as highly innovative and comprise a meaningful portion of new drug approvals.[7] Orphan drugs may qualify as breakthrough therapies.
Such measures of innovation can be helpful to an extent. Still, they obscure the actual rate of innovation since they consider the results as opposed to the methodology applied to yield the results. If a subsequent breakthrough therapy or an orphan drug, for example, delivers what is determined to be a limited improvement to existing treatments, this may encourage a skewed innovation score. In addition, a subsequent orphan or first-in-class drug in any given therapeutic area may be classified as a follow-on drug, despite being highly innovative.
A new measure of innovation for small molecules and peptide drugs based on structural novelty is proposed by Wills & Lipkus (2020): Structural Approach to Assessing the Innovativeness of New Drugs Finds Accelerating Rate of Innovation.[8] Wills and Lipkus infer a marked increase in innovation in the above-mentioned drug categories over the last several decades, represented in Figure 1 below.
Molecular Shape Impacts Medicinal Properties
Because chemical structure and pharmacological activity are related, examining the molecular structure of a drug is a valuable lens through which innovation can be evaluated.
STRUCTURAL INNOVATION MATRIX
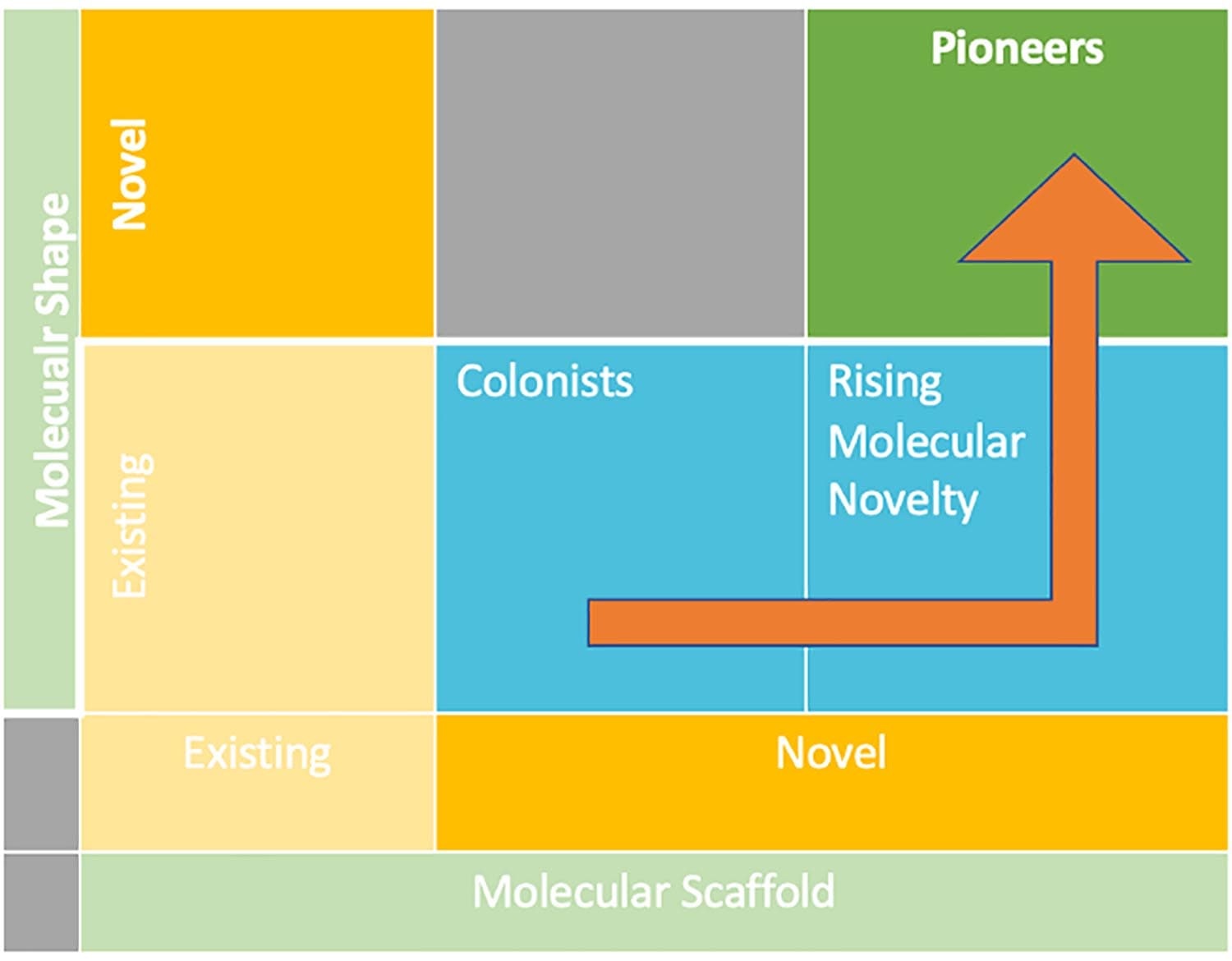
Figure 1: Structural Innovation Matrix adapted from Wills & Lipkus, (2020)
Small-molecule drugs act by binding to specific proteins originally destined for particular molecules produced in the body naturally. Molecular structure affects a drug’s ability to attach to the desired binding site and take its pharmacological effect. For example, in cancer therapy, these small-molecule inhibitors interrupt various protein pathways, which can result in decreased multiplication of cancer cells.
Scaffolds
The molecular scaffold is one of the most widely applied representations in medicinal chemistry and drug design. This concept is used extensively in medicinal chemistry and drug design to generate, study, and compare the core structures of active compounds and can be a powerful indicator of innovation in small molecules.
In drug discovery, scaffolds that exhibit structural diversity and favourable bioactivity properties are in the highest demand. They carry the strongest potential for identifying treatments that can either be immediately synthesised or provide the baseline for progressing existing treatments into novel drugs.
Molecular scaffolds can be derived by removing atoms from side chains, as described initially by Bemis and Murcko.[9] These frameworks based on the original compound are created by progressively removing univalent atoms until only ring and linker atoms remain.[10]
As shown below in Figure 2, specific chemical features (i.e., the types of elements and bonds) can be ignored, and the scaffold can be deconstructed further. When an innovative scaffold is discovered and deconstructed, opportunities open to functionalise it in new ways to create a molecule which is a cut above in terms of activity.
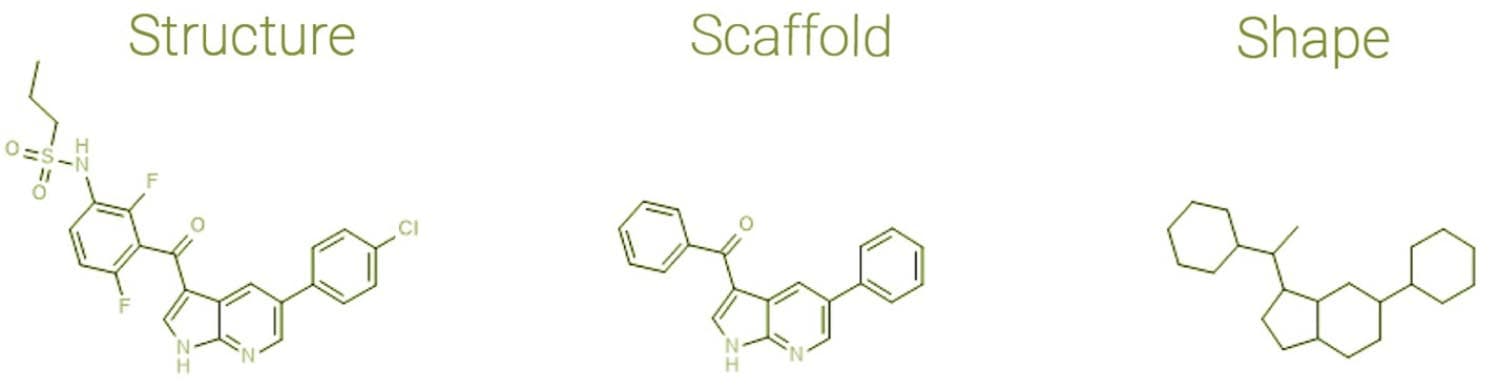
Figure 2: Deriving Scaffolds and Shapes from Molecules. Adapted from Bournez C, Carles F, Peyrat G, Aci-Sèche S, Bourg S, Meyer C, Bonnet P 2020[11]
Conclusion
The drug pipeline continues to be driven by small molecules. With 18 novel agents approved, cancer drugs represented the lion’s share of new drug approvals in 2020, with small molecules contributing to nearly all innovative treatments in this segment.
Along with their inherent advantages of flexibility of action and adaptability in administration, small molecules can be combined with other medications, thus increasing clinical effects beyond mono-targeted treatment options. As a result of their long shelf-life and frequent oral bioavailability, small molecules can be available to more patients than any other therapeutic modality. Thereby, their application is highly sustainable for healthcare systems. It is predicted that small molecules will continue to drive drug research and improve the lives of patients in the future.
On a broader scale, structure-based analysis of drug innovation indicates that pioneers have been trending upward in FDA-approved drugs over the past couple of decades. Based on 2020 drug approvals, healthcare innovators are expected to continue their pursuit of structural innovation in their quest to develop new and better medications with improved patient outcomes, discover treatments for currently incurable conditions, and secure patent protection for new, groundbreaking therapies.
[1] Jones A. W. (2011). Early drug discovery and the rise of pharmaceutical chemistry. Drug testing and analysis, 3(6), 337–344. https://doi.org/10.1002/dta.301
[2] Hartmut Beck, Michael Härter, Bastian Haß, Carsten Schmeck, Lars Baerfacker, Small molecules and their impact in drug discovery: A perspective on the occasion of the 125th anniversary of the Bayer Chemical Research Laboratory, Drug Discovery Today,Volume 27, Issue 6, 2022,Pages 1560-1574, ISSN 1359-6446
[3] Cristina Mendonça Nogueira T, Vinicius Nora de Souza M. New FDA oncology small molecule drugs approvals in 2020: Mechanism of action and clinical applications. Bioorg Med Chem. 2021;46:116340. doi:10.1016/j.bmc.2021.116340
[4] Lipkus, A. (n.d.). Drugs approved by the FDA in 2020 reflect continuing trend of structural innovation. CAS. https://www.cas.org/resource/blog/drugs-approved-fda
[5]Center for Drug Evaluation and Research. (2022, February 3). Frequently Asked Questions: Breakthrough Therapies. U.S. Food And Drug Administration. https://www.fda.gov/regulatory-information/food-and-drug-administration-safety-and-innovation-act-fdasia/frequently-asked-questions-breakthrough-therapies
[6] Kepplinger EE (February 2015). “FDA’s Expedited Approval Mechanisms for New Drug Products”. Biotechnol Law Rep. 34 (1): 15–37. doi:10.1089/blr.2015.9999. PMC 4326266. PMID 25713472.
[7] Lanthier, Michael; Miller, Kathleen L.; Nardinelli, Clark; Woodcock, Janet (2013-08-01). “An Improved Approach To Measuring Drug Innovation Finds Steady Rates Of First-In-Class Pharmaceuticals, 1987–2011”. Health Affairs. 32 (8): 1433–1439. doi:10.1377/hlthaff.2012.0541. ISSN 0278-2715. PMID 23918488
[8]Wills, T. J., & Lipkus, A. H. (2020). Structural Approach to Assessing the Innovativeness of New Drugs Finds Accelerating Rate of Innovation. ACS Medicinal Chemistry Letters.
https://doi.org/10.1021/acsmedchemlett.0c00319
[9] Exploratory Data Analysis With mols2grid and Bemis-Murcko Frameworks. (n.d.). Exploratory Data Analysis with Mols2grid and Bemis-Murcko Frameworks. Retrieved November 16, 2022, from http://practicalcheminformatics.blogspot.com/2021/10/exploratory-data-analysis-with.html
[10] Bemis, G. W., & Murcko, M. A. (1996). The Properties of Known Drugs. 1. Molecular Frameworks. Journal of Medicinal Chemistry, 39(15), 2887–2893. https://doi.org/10.1021/jm9602928
[11] Bournez, C., Carles, F., Peyrat, G., Aci-Sèche, S., Bourg, S., Meyer, C., & Bonnet, P. (2020). Comparative Assessment of Protein Kinase Inhibitors in Public Databases and in PKIDB. Molecules, 25(14), 3226. https://doi.org/10.3390/molecules25143226
[12] Exploratory Data Analysis With mols2grid and Bemis-Murcko Frameworks. (n.d.). Exploratory Data Analysis with Mols2grid and Bemis-Murcko Frameworks. Retrieved November 16, 2022, from http://practicalcheminformatics.blogspot.com/2021/10/exploratory-data-analysis-with.html
https://www.mdpi.com/1420-3049/25/14/3226/htm
https://pubs.acs.org/doi/pdf/10.1021/acs.jmedchem.5b01746
https://media.nature.com/original/magazine-assets/d41573-021-00002-0/d41573-021-00002-0.pdf
https://www.cas.org/resource/blog/drugs-approved-fda
https://docs.chemaxon.com/display/docs/bemis-murcko-clustering.md
Author: Lara Bartlett, Digital Communications, Marketing & PR, KVALITO Consulting Group
KVALITO is a strategic partner and global quality and compliance service and network for regulated industries. To find out more, please visit us at www.kvalito.ch. If you would like to benefit from KVALITO’s expert services, please send us an email at contact@kvalito.ch. Are you looking for an exciting and challenging position as a consultant, or you are an ambitious student/graduate looking for an internship? Please send your complete application to recruiting@kvalito.ch.