KVALITO’s expertise in project management, Global Clinical Supply systems, and Interactive Response Technology enabled the seamless onboarding and integration of a second IRT vendor and the decommissioning of a legacy application, resolving historical challenges and positioning the client for greater efficiency, flexibility, and scalability in clinical supply operations.
Background
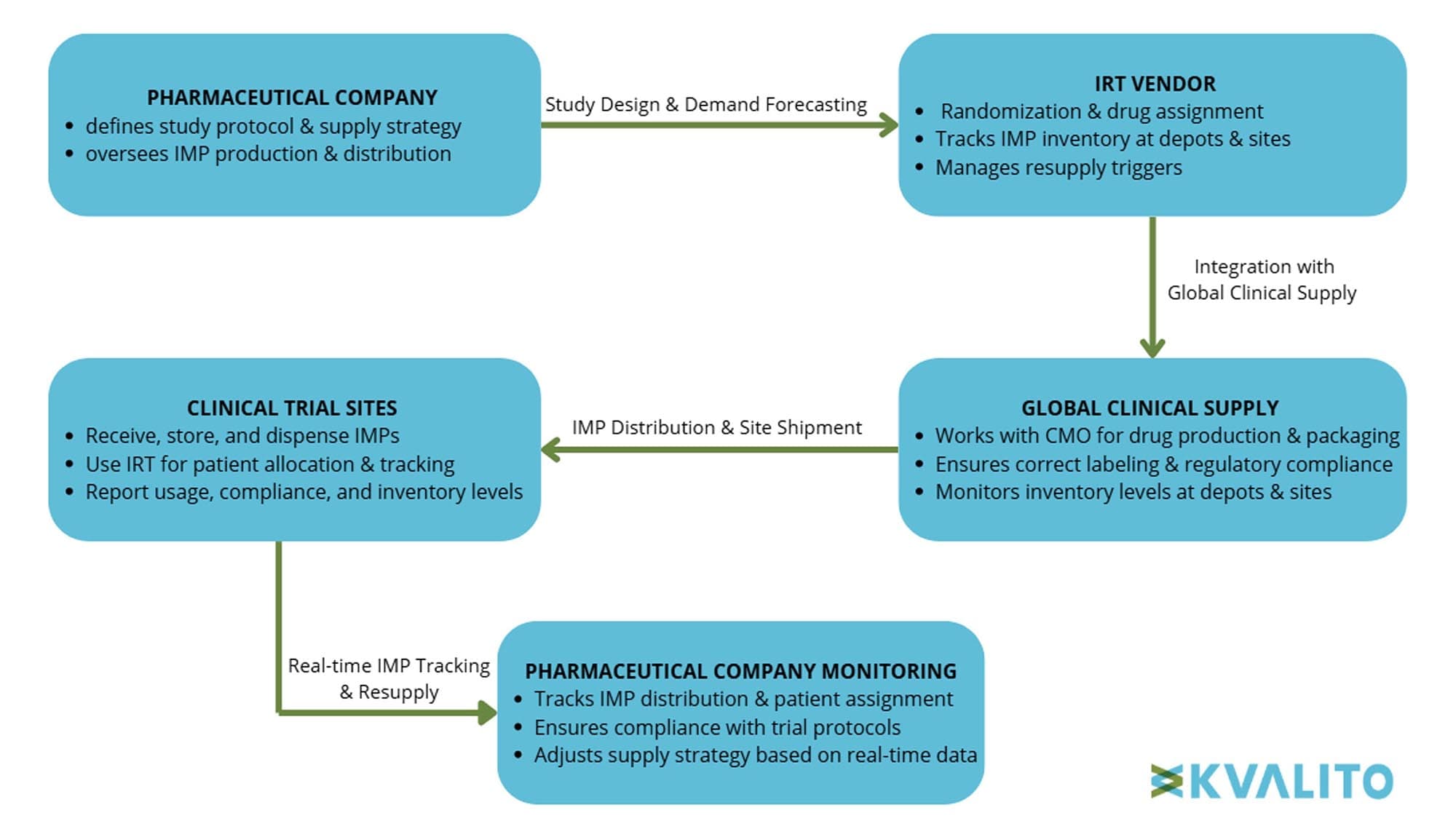
Interactive Response Technology (IRT) systems are critical for managing clinical supply operations, ensuring that investigational medicinal products (IMPs), which are pre-drugs not yet approved for market use, are effectively allocated and tracked throughout clinical trials. In the context of the Global Clinical Supply (GCS) framework, IRT serves as the backbone for supply chain orchestration, integrating with multiple clinical and supply systems to manage enrollment, drug dispensing, re-supply strategies, and blinding processes. IRT also enables real-time tracking of investigational products, ensuring compliance with protocol-specific dosing regimens and regulatory requirements, including adherence to ICH-GCP (International Council for Harmonization – Good Clinical Practice), FDA 21 CFR Part 11, and EMA guidelines for electronic records and data integrity while supporting adaptive trial designs and demand-driven supply planning.
These systems facilitate key functionalities such as patient and treatment randomization, drug allocation, and inventory tracking, ensuring unbiased treatment assignment and adherence to study protocols. Within the broader GCS framework, IRT systems function as an integral component for ensuring seamless coordination between clinical trials, supply chain logistics, and compliance with regulatory standards.
Figure 1: Pharmaceutical company & IRT vendor in GCS for IMP delivery, Copyright KVALITO Consulting Group, 2025
Challenge
Key challenges in Integrated Clinical Supply systems include real-time and batch services, Computerized System Validation (CSV) under Good Clinical Practice (GCP), integration of multiple systems, data integrity, and timely supply management. Additionally, collaborating with third-party vendors introduces complexities such as ensuring seamless interoperability between vendor systems, maintaining compliance across different service providers, and managing communication protocols to guarantee data integrity and regulatory adherence.
The client had historically relied on a single IRT vendor to manage these critical processes. While effective in the past, this dependency posed significant operational and strategic risks. A single-vendor strategy limited the client’s flexibility to adapt to changing trial demands, created potential vulnerabilities in the event of vendor issues, and constrained scalability for increasingly complex global clinical trials.
To address these challenges, the client initiated a strategic decision to:
- Onboard a second IRT vendor into their clinical supply systems landscape.
- Decommission a legacy application, which had limited functionality and high operational costs.
This dual-vendor approach and application decommissioning were designed to:
- Enhance operational flexibility and scalability.
- Mitigate risks associated with single-vendor reliance.
- Strengthen the ability to support increasingly complex and adaptive clinical trial designs.
- Simplify the system landscape for future programs such as Intelligent Clinical Supply Management (ICSM).
- Reduce IRT operational costs by eliminating an application with limited functionality, no archival data requirements, and minimal business benefit.
The application decommissioning effort presented its own set of challenges, including:
- Managing the integrated systems connectivity during decommissioning.
- Ensuring GxP compliance while retiring a high-risk application.
- Executing the decommissioning without archival due to the absence of source data.
Successfully addressing these complexities required meticulous planning, execution, and validation to achieve a robust, compliant, and future-ready system landscape.
What KVALITO did
Part 1: Integration and Hyper Care of the Second IRT Vendor
KVALITO supported the project by stabilizing and optimizing the end-to-end (E2E) GxP system integration of the second IRT vendor into the client’s GCS systems. This involved ensuring seamless system functionality, compliance, and operational readiness throughout the hyper care phase. The E2E system integration included six key downstream applications, encompassing supply systems for real-time inventory alignment, compliance systems to maintain regulatory and quality adherence, data analytics platforms for advanced reporting and predictive insights, regulatory systems to enforce global compliance standards, and clinical program systems to ensure seamless trial execution.The first study under this dual-vendor setup was fully integrated and successfully navigated all GCS phases, marking a significant milestone in the client’s clinical supply operations. Our team ensured compliance, functionality, and performance requirements were met while supporting critical milestones and delivering long-term value.
One of the most critical aspects of onboarding a new vendor within an integrated landscape is ensuring seamless data mapping, consistency, and system stability. Given the complexity of integrating an IRT vendor with the client’s GCS systems, any discrepancies—such as unexpected system behaviors, format mismatches, or integration errors—could lead to compliance risks, data integrity issues, or operational disruptions.
To ensure smooth integration and system reliability, a structured approach was implemented, consisting of two key phases: Development & Integration and Hyper Care.
Development & Integration
- Proactive Data Mapping – Collect and align IRT and GCS Integration Specifications to ensure seamless data flow.
- CSV System Validation & End-to-End Testing – Perform CSV and conduct comprehensive E2E integrated system landscape testing.
Hyper Care
- Enhancements & Root Cause Analysis (RCA) – Implement system enhancements and conduct thorough root cause analysis to address integration gaps.
- Ensuring System Reliability & Study Readiness – Validate system stability, performance, and compliance before full deployment.
1. Proactive Data Mapping (Development & Integration)
A comprehensive data mapping exercise was conducted to align IRT vendor data requirements (data links) with client applications. To prevent discrepancies, rigorous data validation checks were established, ensuring no typos, format inconsistencies, or misaligned data structures across systems.
A detailed Integration Specification document was developed to outline the systems involved in data exchange, ensuring clarity in data flow processes. This specification included:
- A system architecture overview, mapping all integration points between IRT and GCS.
- A list of critical data elements, specifying data format, field lengths, and transformation rules to maintain consistency across platforms.
- Data flow diagrams, illustrating the movement of data between systems and identifying key checkpoints for validation.
- Error overseeing protocols, defining measures to detect, log, and resolve data discrepancies in real-time.
- Security and compliance considerations, ensuring adherence to regulatory industry-specific guidelines.
- Ongoing governance strategy, specifying version control, audit trails, and continuous improvements to maintain system alignment as business requirements evolve.
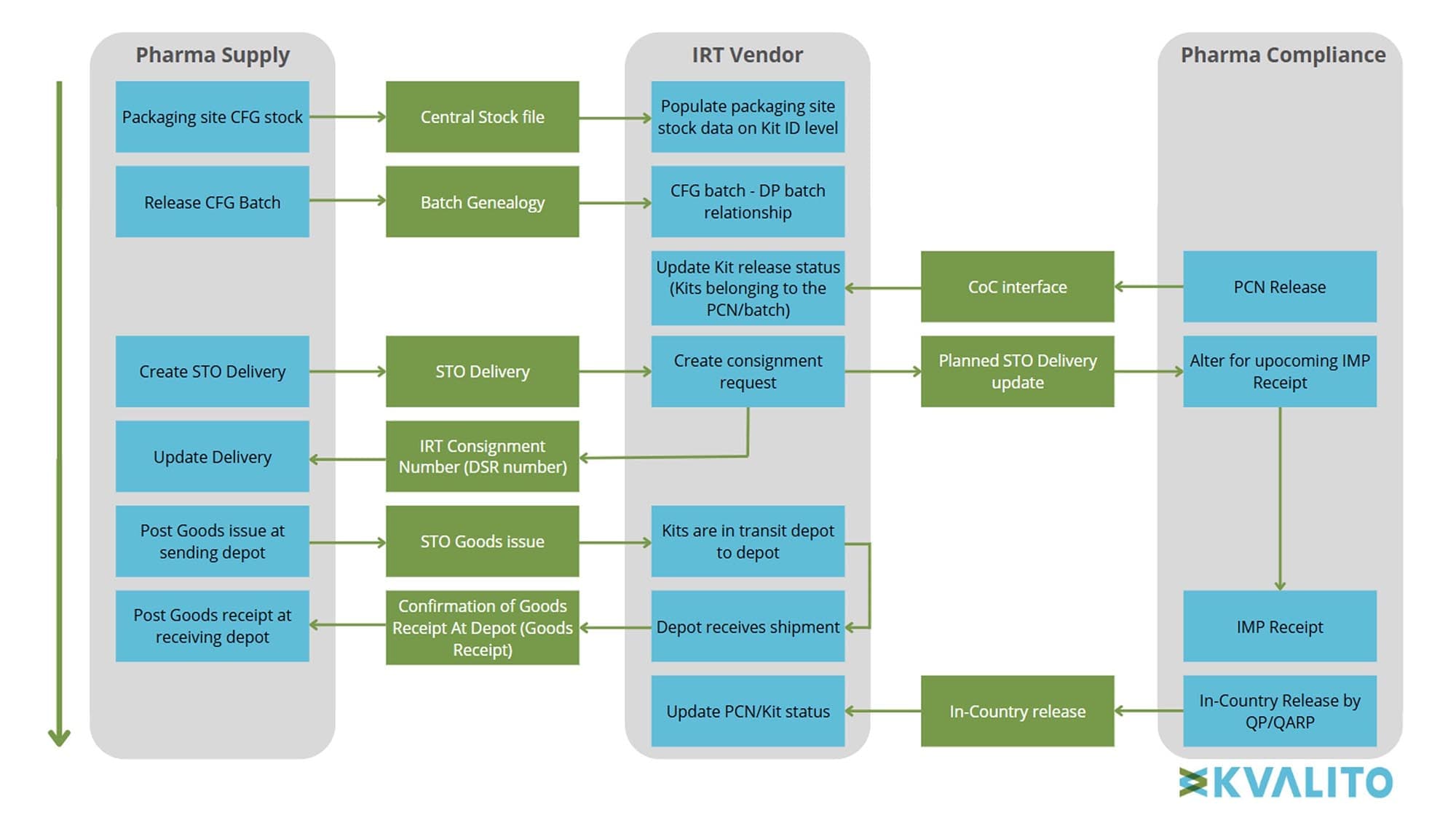
This specification served as a foundational reference, providing both technical and business teams with a clear understanding of data interactions and ensuring seamless integration across platforms. Additionally, regular monitoring was conducted with technical SMEs and business stakeholders to track data flows and verify seamless message transitions across integration points.
Figure 2: End-to-End Message Flow: From Batch Release to In-Country Release, Copyright KVALITO Consulting Group, 2025
2. CSV System Validation & End-to-End Testing (Development & Integration)
As part of the integration and onboarding of the IRT vendor, system validation and comprehensive testing were conducted to ensure alignment between the client’s (pharma company) systems and the IRT platform. Both parties were involved in development efforts, followed by rigorous validation and testing phases to confirm system functionality, compliance, and readiness for deployment.
Formal Validation, Testing Phases & Technical Go-Live
A structured CSV approach was followed, adhering to industry best practices and regulatory requirements. The testing was executed in multiple phases:
System-Level Testing (IQ, OQ, PQ) in QA Environment
- Installation Qualification (IQ): Verified that system components were installed correctly and met predefined technical specifications.
- Operational Qualification (OQ): Assessed whether the system performed as expected under normal operating conditions.
- Performance Qualification (PQ): Ensured the system consistently met performance criteria and business requirements.
E2E Testing in the Integrated Landscape
- E2E Operational Qualification (E2E OQ): Focused on validating system interactions across all integrated components, ensuring seamless data exchange and workflow execution.
- E2E Performance Qualification (E2E PQ) & User Acceptance Testing (UAT): Conducted in collaboration with business users to validate real-world scenarios, confirm regulatory compliance, and assess overall system usability.
Following successful validation and UAT approval, a technical go-live was executed to transition the integrated IRT system into production. This was followed by the first fully integrated IRT vendor study go-live, marking a critical milestone in the system’s deployment. Real-time monitoring and post-go-live validation ensured a smooth transition, with contingency plans in place to mitigate potential risks. With the system now live, the Hyper Care phase began, providing enhanced support and monitoring to ensure stability and address any early-stage issues.
3. Enhancements and Root Cause Analysis (RCA) (Hyper Care)
A structured RCA and enhancement process was followed to address discrepancies between expected and actual system outcomes, ensuring long-term system stability and performance improvements. Each identified issue was assessed, resolved, and implemented as an enhancement to strengthen the IRT solution.
RCA: Conducted in-depth investigations of system errors by analyzing logs, data flows, and application interactions as well as clearly defining ownership of the incident, ensuring responsible parties (vendor, internal IT, or business teams) took accountability for resolution.
Impact Assessment: Assessed downstream and upstream effects across integrated applications to ensure correct allocation of issue resolution responsibilities.
Integration Specification Update: Collaborated with the vendor to update the Integration Specification Document as part of the GxP change management process, ensuring that all system modifications adhered to regulatory requirements, quality standards, and operational best practices. This involved:
- Impact Assessment: Evaluated potential system, process, and compliance impacts before implementing changes.
- Change Control & Documentation: Ensured that updates were governed by a structured change control process, including change request approvals, impact analysis, and risk assessments.
- Regulatory Compliance & Traceability: Maintained full traceability of changes in accordance with GxP guidelines, ensuring proper documentation for regulatory audits and inspections.
- Stakeholder Alignment: Worked closely with business, IT, and QA teams to ensure that all updates met operational requirements and regulatory expectations.
- Validation & Approval: Conducted formal validation activities to confirm that changes did not introduce new risks or system discrepancies before approval and deployment into production.
Enhancement Implementation and Testing: Deployed and validated enhancements by conducting UAT in a QA environment (E2E testing setup) in PROTON mirroring the production (PROD) system, validating that the fix resolved the issue and did not introduce new defects before moving to production.
Production Deployment and Verification: Successfully pushed enhancements to PROD only after passing rigorous QA validation. Verified that the original RCA findings were resolved in a live environment, ensuring operational stability before moving forward with the next clinical study milestone.
Ongoing System Optimization: Successfully delivered eleven targeted enhancements to address functional gaps, improve system performance, and ensure the IRT solution fully met operational needs.
Seamless Deployment Support: Monitored post-deployment performance and collaborated with stakeholders to ensure smooth integration within the clinical supply landscape.
4. Ensuring System Reliability and clinical study Readiness (Hyper Care)
Through this structured approach, system integrity was maintained, ensuring that critical messages successfully traversed all phases, reaching in-country drug release without discrepancies.
Collaboration with stakeholders was essential to address outstanding issues, ensuring a stable and compliant system environment. This methodology not only resolved immediate issues but also enhanced system resilience, ensuring that the dual-vendor integration remained compliant, stable, and scalable for future studies.
KVALITO played a key role in ensuring the successful integration of the second IRT vendor, contributing to:
- Facilitating the successful go-live of the second vendor’s first clinical study, marking a significant achievement in the dual-vendor integration strategy.
- Supporting the First Patient First Visit (FPFV) milestone and the in-country release in multiple countries globally by ensuring the system was fully prepared for clinical trials without delays.
- Verifying system readiness to meet clinical trial demands, leading to the completion of the hyper care phase and enabling a stable handover to routine operations.
Part 2: Application Decommissioning
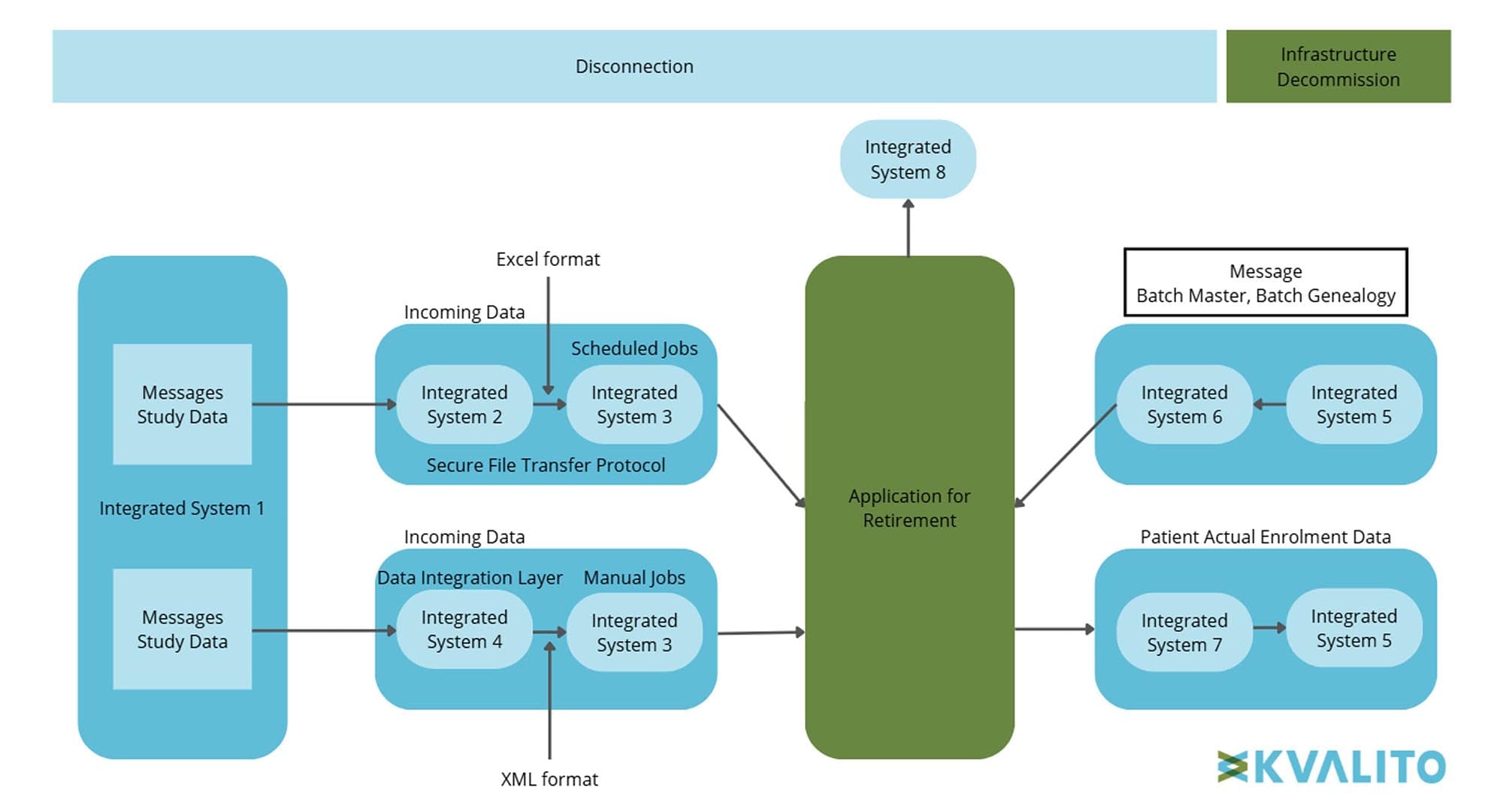
The decommissioning effort focused on retiring a legacy GxP high application that had limited functionality, high operational costs, and minimal business value. The application supported only one clinical study and did not contain source data, eliminating the need for archival. This initiative aimed to simplify the system landscape for future ICSM programs while reducing IRT operational costs. Given the application’s high-risk classification, the decommissioning required careful management of integrated system connectivity to prevent disruptions and strict adherence to GxP compliance requirements.
Figure 3: Data flow overview, Copyright KVALITO Consulting Group, 2025
To ensure a structured and compliant approach, best-in-class project management activities were followed, including the creation of a project charter, and guiding the workstream through tollgates. The process adhered to the IT Waterfall CSV methodology, aligning with GAMP5 best practices to maintain regulatory compliance throughout the decommissioning lifecycle.
The decommissioning workstream was structured into three key stages:
- Retirement Plan
This phase focused on defining the scope, establishing timelines, and securing approvals for the retirement strategy. Key activities included:
-
- Project Kick-off – Initiated the decommissioning effort with stakeholder alignment.
- Tollgate Scope and Seek Approval – Passed the required governance checkpoints to proceed.
- Impact Assessments – Conducted a Change Impact Assessment (CIA) to evaluate dependencies and risks, ensuring a smooth transition.
- Regulatory and QA Alignment – Collaborated with QA and compliance teams to confirm adherence to GxP and validation requirements.
- Retirement Plan Development and Approved – Formalized the approach for execution, detailing system decommissioning steps, compliance measures, and risk mitigation strategies.
- Retirement Execution
This phase focused on systematically retiring the application and its associated components while ensuring compliance and minimizing disruption. Key activities included:
-
- Service & Contract Terminations – Canceled/terminated service agreements related to system operation and software maintenance.
- Project Documentation Archival – Archived documentation from the initial implementation of the application/IT system.
- System Component Decommissioning – Deactivated or decommissioned related system components, including:
- Executing the archival of source code in Bitbucket.
- Deactivating Development and QA databases.
- Making obsolete system-specific deliverables from the PROTON Domain.
- Retiring SOPs and FRMs related to access and usage of data.
- Stopping message transmissions from interconnected systems to the retiring system.
- QA Activities – Ensured compliance throughout the execution phase while updating the CIA document with QA/test details.
- De-connectivity in Production – Disconnected interconnected systems one by one to prevent data inconsistencies or disruptions.
- IT Infrastructure Retirement – Finalized system decommissioning by retiring the associated IT infrastructure components.
- Reporting Phase
The final phase ensured a documented and compliant closure of the decommissioning process. Key activities included:
-
- Final Documentation & Compliance Review – Verified and documented all decommissioning activities for audit and regulatory purposes.
- Final Execution Approvals – Obtained necessary approvals to confirm the successful decommissioning of the application.
- Project Records Archival – Archived project-related records to complete the decommissioning process in compliance with regulatory and business requirements.
People, Processes and Tools
People / Roles
- IRT Project Manager
- Project Quality Manager
- Business Project Manager
- Application Manager
- eCompliance Manager
- Technical System Owner
- Business System Owner
- Service Transition Expert
- Business Information Security Manager/Expert
Processes
Business Process Mapping: Prior to onboarding and integrating the IRT vendor, a comprehensive business process mapping exercise was conducted. This effort aimed to document existing workflows, identify optimization opportunities, and align processes with the future state of the integrated IRT platform. By proactively mapping these processes, potential gaps and inefficiencies were addressed, ensuring a smoother transition and minimizing operational disruptions.
Waterfall Project Management Approach: A structured phase-gated methodology was applied, allowing clear tracking of milestones from initiation to completion.
Regulatory Compliance and Validation (CSV under GxP): Ensured all activities adhered to GAMP5 best practices, including:
- Validation planning and execution to confirm system compliance.
- Risk-based approach to testing and validation aligned with industry guidelines.
- Change management controls (via SNOW) to ensure traceability of modifications.
Issue and Risk Management: A proactive approach was applied to monitor potential project risks, establish mitigation strategies, and minimize impact on operations.
Tools and Technologies
Project Management and Collaboration: MS Project, MS SharePoint, MS Teams, MS PowerPoint, MS Excel, MS Visio, Outlook.
Validation and Compliance Tools: validated document management system,
Change Management: Configuration Management Database ServiceNow
Value Delivered
Seamless Onboarding and Integration
Successfully onboarded and integrated the second IRT vendor into the client’s GCS systems, enhancing operational flexibility and supply chain resilience.
Enhanced Clinical Trial Efficiency
Enabled the FPFV milestone by ensuring the integrated system was ready to support clinical trials without delays.
Risk Mitigation
Resolved historical risks associated with a single-vendor strategy by implementing a robust dual-vendor setup, delivering an enhanced IRT platform for the client.
Validated and Compliant Execution
Successfully delivered GDPs-compliant system integration and application decommissioning, meeting ICH-GCP, FDA 21 CFR Part 11, and EMA regulations.
Enhanced Project Execution
Applied structured project management methodologies, ensuring timely milestone delivery, financial oversight, and quality assurance.
Sustainable Framework for Future Growth
Established scalable processes and governance models for future system enhancements and clinical supply improvements.
Stakeholder Communication and Collaboration
A key success factor was the efficient coordination between multiple stakeholders across different functions, ensuring smooth execution of both the integration and decommissioning workstreams. KVALITO facilitated this by:
Financial Oversight and Reporting
Budget control and cost-effectiveness were essential in managing the project’s financial performance while ensuring resource optimization. The project team executed:
Cost-benefit analysis for decommissioning: Provided data-driven insights into cost reductions achieved through system decommissioning, helping stakeholders quantify the financial impact of removing the legacy application.
GDPs, IT, and Clinical Project Management
Effective project management was critical to the successful execution of the IRT vendor integration and the application decommissioning initiative. KVALITO applied structured project management methodologies, robust stakeholder coordination, financial oversight, and adherence to regulatory compliance to ensure seamless execution and long-term sustainability.
References:
- Interactive Response Technology (IRT)
ICH E6 (R2) Good Clinical Practice (GCP) Guidelines
https://www.ich.org/page/efficacy-guidelines
Good Clinical Practice Directive 2005/28/EC of 8 April 2005
Directive – 2005/28 – EN – EUR-Lex
Clinical Trials Transformation Initiative (CTTI)
https://www.ctti-clinicaltrials.org/
- Global Clinical Supply (GCS)
Global Clinical Supplies Group (GCSG)
https://mygcsg.com/
PPD Clinical Supply Management Best Practices
https://www.ppd.com/our-solutions/clinical/phase-ii-iv-clinical-trial-management/supplies/
- Computerized System Validation (CSV) in GxP Environments
ISPE Good Automated Manufacturing Practice (GAMP) 5 Guidelines
https://ispe.org/
FDA 21 CFR Part 11 – Electronic Records & Signatures
https://www.ecfr.gov/current/title-21
European Medicines Agency (EMA) – EudraLex Volume 4, Annex 11 (Computerized Systems)
https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-computerised-systems-electronic-data-processing_en.pdf
ISPE GAMP 5 – Validation Framework
https://ispe.org/publications/guidance-documents/gamp-5-guide-2nd-edition
- Adaptive Trial Designs & Demand-Driven Supply Planning
FDA: “Adaptive Designs for Clinical Trials of Drugs and Biologics” (2019)
https://www.fda.gov/media/78495/download
EMA: “Reflection Paper on Methodological Issues in Adaptive Clinical Trials”
https://www.ema.europa.eu/en/human-regulatory/research-development/scientific-guidelines/methodological-guidelines/adaptive-clinical-trials
ICH E9 (R1) Statistical Principles for Adaptive Design in Clinical Trials
https://www.ich.org/page/efficacy-guidelines
- Clinical Supply Chain & Investigational Medicinal Products (IMP) Management
WHO Guidelines on GMP for Investigational Products
https://www.who.int/publications-detail/qa-good-manufacturing-practice-for-investigational-products
ISPE: Clinical Trial Supply Chain Management & Best Practices
https://ispe.org/
European Commission: EudraLex Volume 10 – Clinical Trials Guidelines
Site suitability form – EudraLex (Volume 10 – Clinical trials guidelines – Set of documents applicable to clinical trials under Regulation EU No 536/2014) – European Commission
- Regulatory Compliance & Data Integrity in IRT/GCS
FDA: “Data Integrity and Compliance with Drug CGMP”
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/data-integrity-and-compliance-drug-cgmp-questions-and-answers
EMA: “Guidelines on Electronic Data Integrity in Clinical Trials”
https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/guideline-computerised-systems-and-electronic-data-clinical-trials_en.pdf