- Definitions from ISO 9000:2005, revised by ISO 9000:2015 Quality management systems – Fundamentals and vocabulary. A
- Verification: Confirmation, through the provision of objective evidence, that specified requirements have been fulfilled.
- Validation: Confirmation, through the provision of objective evidence, that the specified requirements for a specific intended use or application have been fulfilled.
- Qualification: Process to demonstrate the ability to fulfi
ll specified requirements
In general :
- Validation: For systems or general topics: process, systems, etc.
- Qualification: For single or individual items within your system, process: Equipment, lab methods, etc
Below is a simplified process diagram on how to validate a system
Different validation approaches exist:
- The traditional approach proceeds with the validation of each unit
- The Continuous process verification in which manufacturing process performance is continuously monitored and evaluated.
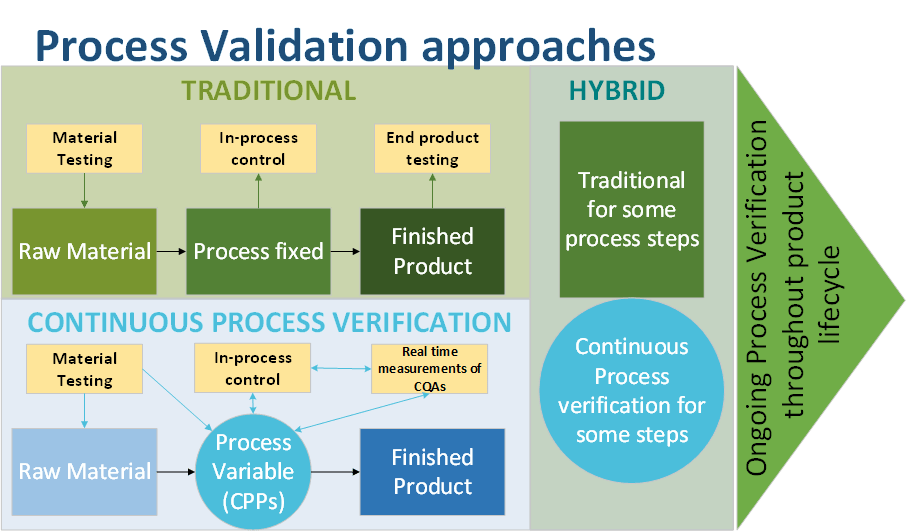
Traditional PV:
- Validationof each unit operation, input/output
- End-product testing
- Process is fixed

Continuous PV:
- Quality by design approach
- Process not fixed but adjustable (endpoint based on Critical Quality Attributes – CQA measurement)
- Continuous monitoring (real-time) of CQAs and CPPs (critical Process Parameters)
- Control Strategy based on PAT
- Batches will have consistent CQAs but vary in actual CPPs values

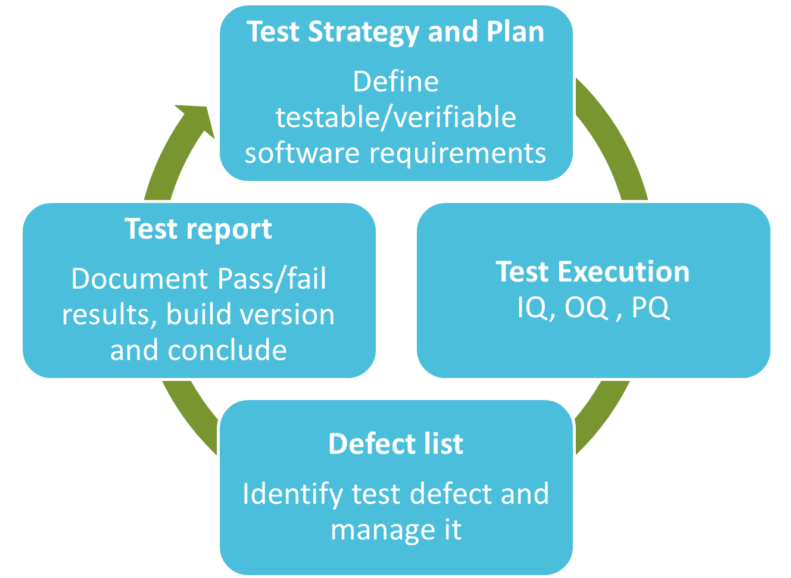
1) A test plan shall be implemented by defining the testable and verifiable software requirements.
2) Testing shall be executed based on Test Strategy and Plan
There are three crucial levels of testing,
- code review,
- unit testing and
- system testing.
Pass or fail results must be documented. In case of a defect, the root cause must be identified, and the risk evaluated. The defect shall be finally resolved.
Defects are unusually documented in a “defect list” during test execution.
Executed testing shall be then documented in a test report. In case defects cannot be resolved during the validation processes,in general defects with low risks, the defects must remain in control and must be managed.




