Table of Contents
These days, research and development of many pharma companies are focused on Cell and Gene Therapies, which bring hope for many patients who suffer from diseases based on genetic disorders like cancer and autoimmune diseases.
But what are cell and gene therapy products, and what materials are they based on?
First of all, we should distinguish between cell therapy products and gene therapy products. Cell therapy products are based on living mammalian cells. Meanwhile, gene therapy products contain nucleic acids (DNA/RNA) as one of their active ingredients. The advantage of these products is that patients’ cells or genes which are dysfunctional or missing can be replaced by engineered cells or genes.
For cell therapy approaches, either cells of human beings (patient’s own cells or cells from another human being) or cells of animal origin such as pigs, primates and cows are used as the starting material.
Gene therapies treat or prevent the progression of genetic disorders by:
- Removing and/or replacing faulty elements of a gene → gene editing
- Preventing the production of a specific protein → gene silencing
- Transferring a full copy of a gene to correct a faulty or missing one → gene transfer
In gene therapies, for example, haemophilia, patients missing a special antihemophilic factor leading to the disease can be treated with the genetic vector carrying the DNA for the “missing” factor (e.g. factor VIII or IX vector). The US Food and Drug Administration (FDA) has recently approved a new drug, Hemgenix, to treat haemophilia. The manufacturer, CSL Behring, has made it the world’s most expensive drug, priced at $3.5 million for a single treatment. Hemgenix is a gene therapy used to treat adults with haemophilia B, which is an inherited bleeding disorder in which people cannot produce the proteins needed to form blood clots.
Ancillary or Raw Materials
The manufacturing of cell and gene-engineered products includes many different reagents and materials like cell-isolation reagents, cell culture media, different chemicals, separation devices and disposables such as plasticware and bioprocessing bags.
All these materials and reagents used as processing and purification aids or agents are defined as ancillary materials (AMs) or raw materials. Even though AM are per definition not part of the final cell and gene product, they must fulfil high-quality standards because they directly affect the product’s stability, safety, potency and purity.
To prevent the introduction of adventitious agents or toxic impurities via cell and gene products into the patient, which can lead to infectious diseases or unwanted immune reactions, it is required to implement a stable qualification process for AM.
AM qualification
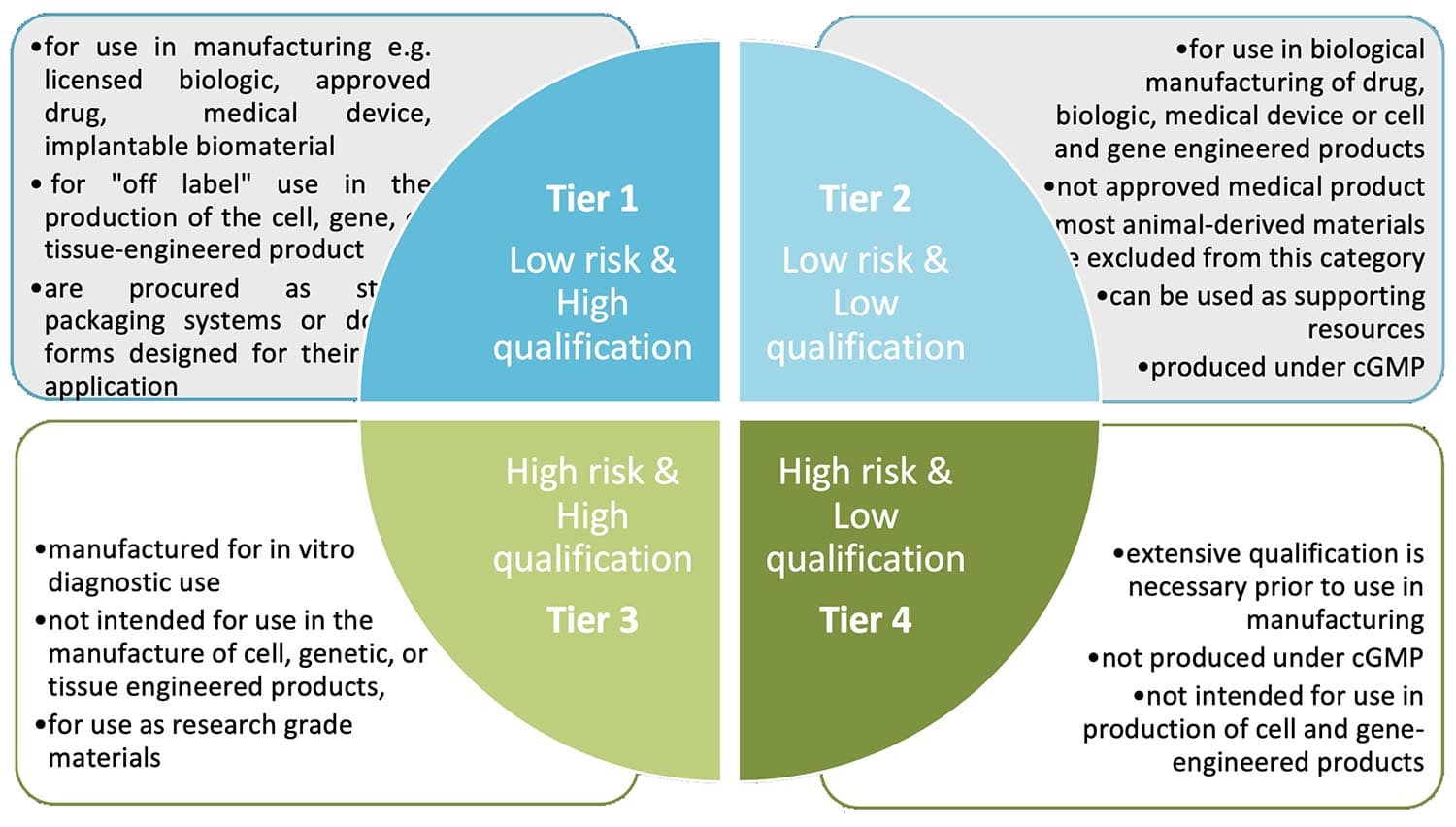
The AM qualification process depends on many varied factors like the type of AM, type of final product and the stage of manufacture in which the AM is used. According to U.S. Pharmacopeia (USP) <1043> “Ancillary Materials for Cell, Gene and Tissue-Engineered Products”, the following steps should be respected as part of AM qualification:
For Tier 1 (low risk, high qualification) manufacturers may need to:
- cross-reference the Drug Master File (DMF),
- obtain a Certificate of Analysis (CoA),
- assess the impact of removal and lot-to-lot variability from the final product,
- conduct stability studies.
For Tier 4 (high risk, minimal qualification) manufacturers must perform all the above activities plus more extensive qualifications like:
- confirming critical CoA results,
- perform random agent tests,
- work with suppliers to update their AM manufacturing process to their current GMP standards.
Emerging Technologies
The future is here: Technologies like CRISPR, CAR-T, and AI-driven personalized medicine are pushing the boundaries of medical science, promising more precise and effective treatments. CRISPR, a powerful gene-editing tool, has shown remarkable potential in correcting genetic defects at their source. The first gene-editing cure is here, and grateful patients are calling it “life-changing.” In just 11 years since the invention of CRISPR, this powerful DNA-editing technology has moved from the lab to clinical application, providing a treatment that effectively addresses the symptoms of sickle-cell disease. CAR-T therapy, which involves modifying a patient’s own T cells to target cancer cells, has provided new hope for patients with certain types of cancer. Furthermore, AI is revolutionizing biomanufacturing and regulatory processes by optimizing production methods, ensuring quality control, and expediting the approval and delivery of these life-changing therapies. These advancements are not only enhancing the efficacy and safety of treatments but also making them more accessible to patients in need.
Conclusion
A good and trustful relationship between AM Supplier and cell and gene therapy manufacturer, including transparent communication, is essential to establish a robust and effective AM qualification system. Choosing already approved AMs, which are manufactured at the supplier under cGMP conditions, can be a smart way for the cell and gene therapy manufacturer to reduce the effort of qualification tests. Even though an AM supplier provides robust quality documentation, the cell and gene therapy manufacturer is always responsible for the AM qualification and must be able to show evidence that the AM fits its intended use, fulfils stability and performance requirements in the specific manufacturing step and that there is no negative impact of the AM on the final product. A risk-based approach for the AM used in the manufacturing of cell and gene products is obligatory to be compliant with current regulations.
Regulations
-USP: USP <1043> Ancillary Materials for Cell, Gene and Tissue-Engineered Products,
-FDA
-GMP
References
STEMCELL Technologies Inc 2019 „Ancillary Material Qualification – A Fundamental Overview” Technical Bulletin
Solomon J et al. (2016) Current perspectives on the use of ancillary materials for the manufacture of cellular therapies. Cytotherapy 18(1): 1–12.
https://cdn.stemcell.com/media/files/techbulletin/TB27066-Ancillary_Material_Qualification.pdf
https://pubmed.ncbi.nlm.nih.gov/26596503/
https://www.drugfuture.com/Pharmacopoeia/USP32/pub/data/v32270/usp32nf27s0_c1043.html
https://cdn.stemcell.com/media/files/techbulletin/TB27066-Ancillary_Material_Qualification.pdf
https://www.isct-cytotherapy.org/action/showPdf?pii=S1465-3249%2815%2901067-1
https://edition.cnn.com/2022/11/23/health/hemophilia-drug-hemgenix/index.html
Photo by Image by pikisuperstar on Freepik
Authors:
Dr. Anna-Lena Hürter, Senior Life Science Consultant (previously KVALITO Consulting Group)
Đurđa Zdravković, Life Science Consultant, KVALITO Consulting Group
KVALITO is a strategic partner, global quality and compliance service, and network for regulated industries. To find out more, please visit us at www.kvalito.ch. If you would like to benefit from KVALITO’s expert services, please send us an email at contact@kvalito.ch. Are you looking for an exciting and challenging position as a consultant, or are you an ambitious student/graduate looking for an internship? Please send your complete application to recruiting@kvalito.ch.