Table of Contents
The German Supply Chain Due Diligence Act (SCDDA) at a Glance
Due Diligence Legal Framework for a Responsible Business Conduct
Requirements for the Corporate Implementation
Consequences on the Pharma Supply Chain
The German Supply Chain Due Diligence Act (SCDDA) at a Glance
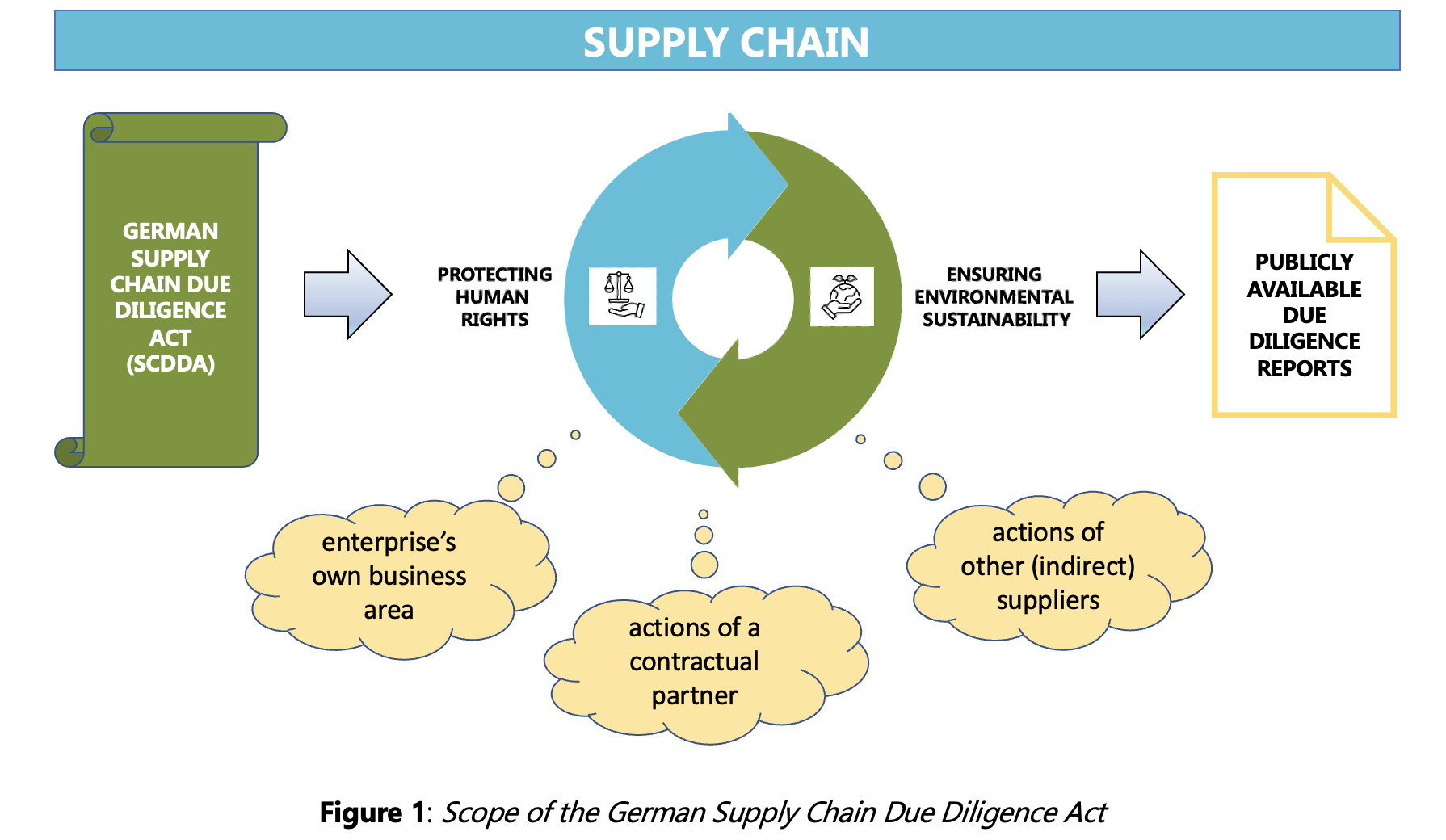
The Act on Corporate Due Diligence Obligations in Supply Chains (also known in Germany as “Lieferkettensorgfaltspflichtengesetz”, or LkSG) came into force on January 1, 2023. This is the first time the responsibility of German enterprises along the entire supply chain has been put on a legal footing with a focus on preventing or minimizing including preventive and remedial measures, together with mandatory complaint procedures.
The SCDDA is binding for all companies with a registered office or subsidiary in Germany and at least 3,000 employees (around 900 companies). In 2024, the scope will be extended to companies with over 1,000 employees.
As a consequence, this new local regulation expands well beyond the German market with a major impact on all the larger companies who predominantly act globally, such as the majority of KVALITO’s clients in the corporate life science and health care industry.
Companies that fail to meet their due diligence and reporting obligations regarding human rights and environmental standards in their supply chains face fines of up to €8 million, depending on the type and severity of the violation. Violators also face reputational damage and could be excluded from the award of public contracts for up to three years.
Due Diligence Legal Framework for a Responsible Business Conduct
In today’s global economy, supply chain due diligence has become a critical aspect of corporate behavior, encompassing the standard of care, prudence, and caution that a reasonable business or person is expected to adhere to while executing or investigating their current supply chain practices in terms of social and environmental processes and policies.
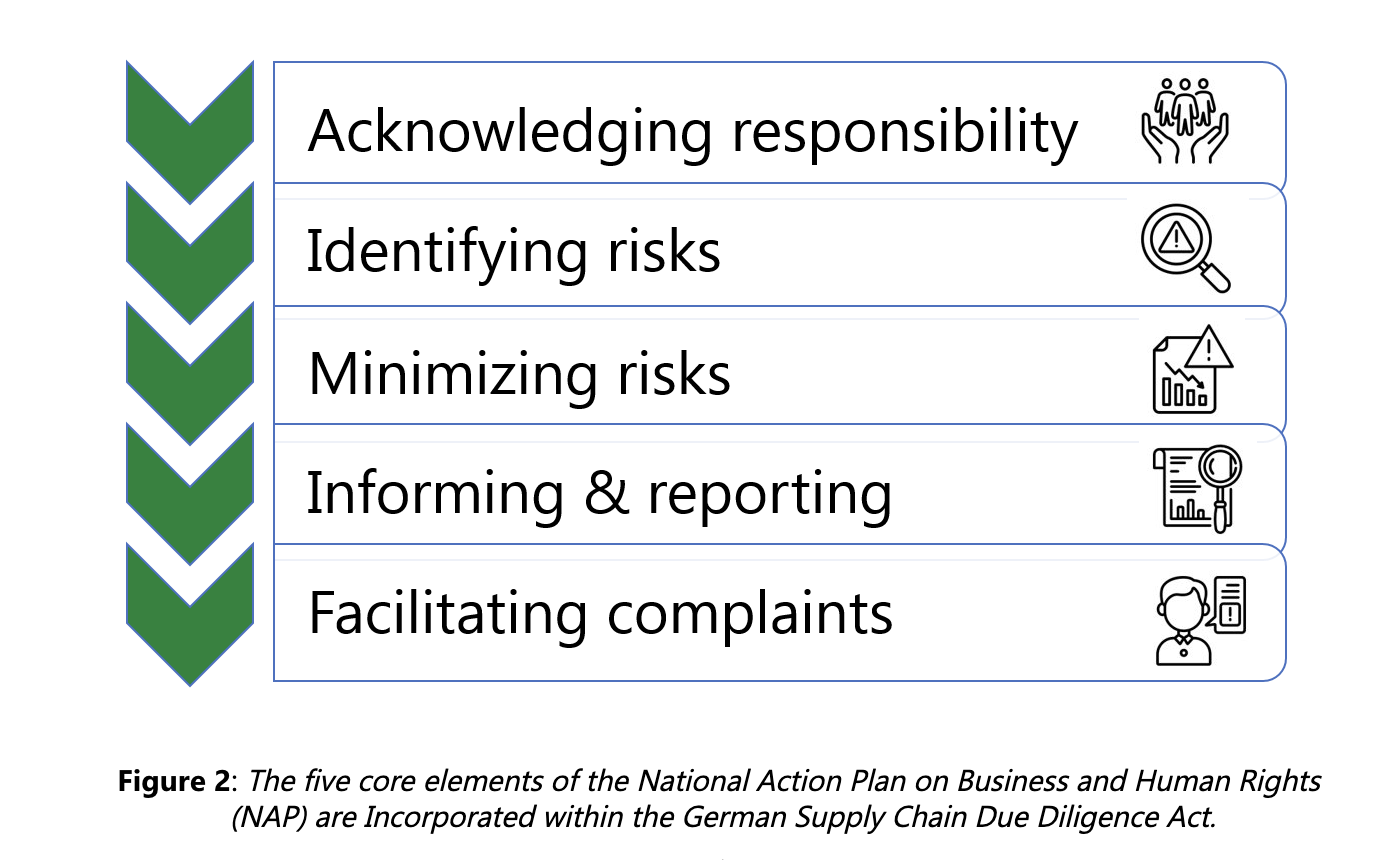
In 2011, the OECD member countries and the United Nations endorsed guiding principles for tougher standards for corporate behavior to cover the steps and processes by which a company understands, monitors and mitigates its As part of this new definition, they utilized a new aspect of due diligence that requires a corporation to investigate third-party partners for potential abuse of human rights: prohibition of child labor, withholding of adequate wage, slavery and forced labor, and denial of access to food and water.
This new aspect of due diligence has gained increasing importance in the context of the UNSDG 2030 Agenda and the 17 sustainable development goals, a global framework adopted by the United Nations in 2015. The goals provide a roadmap for achieving sustainable development globally by 2030.
As mentioned above, the German Supply Chain Due Diligence Act (SCDDA), which entered into force on January 1, 2023, is a piece of legislation aimed at safeguarding human rights and the environment in the global economy more effectively. The SCDDA aligns with several of the UNSDG goals, specifically SDG 8 (Decent Work and Economic Growth), SDG 12 (Responsible Consumption and Production), and SDG 16 (Peace, Justice, and Strong Institutions; establishing compliance procedures and due diligence processes to increase accountability in supply chains), and goal 17 (Partnership for the Goals, requiring companies to establish and maintain partnerships with their suppliers to ensure compliance with human rights, environmental standards and sustainable development).
The German SCDDA extends the understanding of due diligence to environmental sustainability as to the expectation of enterprises to prevent or mitigate adverse environmental impacts that are directly linked to their business operations, even if they do not contribute to those impacts. There is a growing emphasis on the need for comprehensive supply chain due diligence that goes beyond traditional risk management to include social and environmental considerations that underpin several global sustainability goals.
It is important to note that while the SCDDA is a significant step towards promoting human rights, sustainability, and responsible business practices, it is part of a broader global effort to achieve the UNSDG 2030 Agenda, requiring collaborative action by governments, businesses, civil society, legislation, and other stakeholders at national, regional, and international levels.
Requirements for the Corporate Implementation
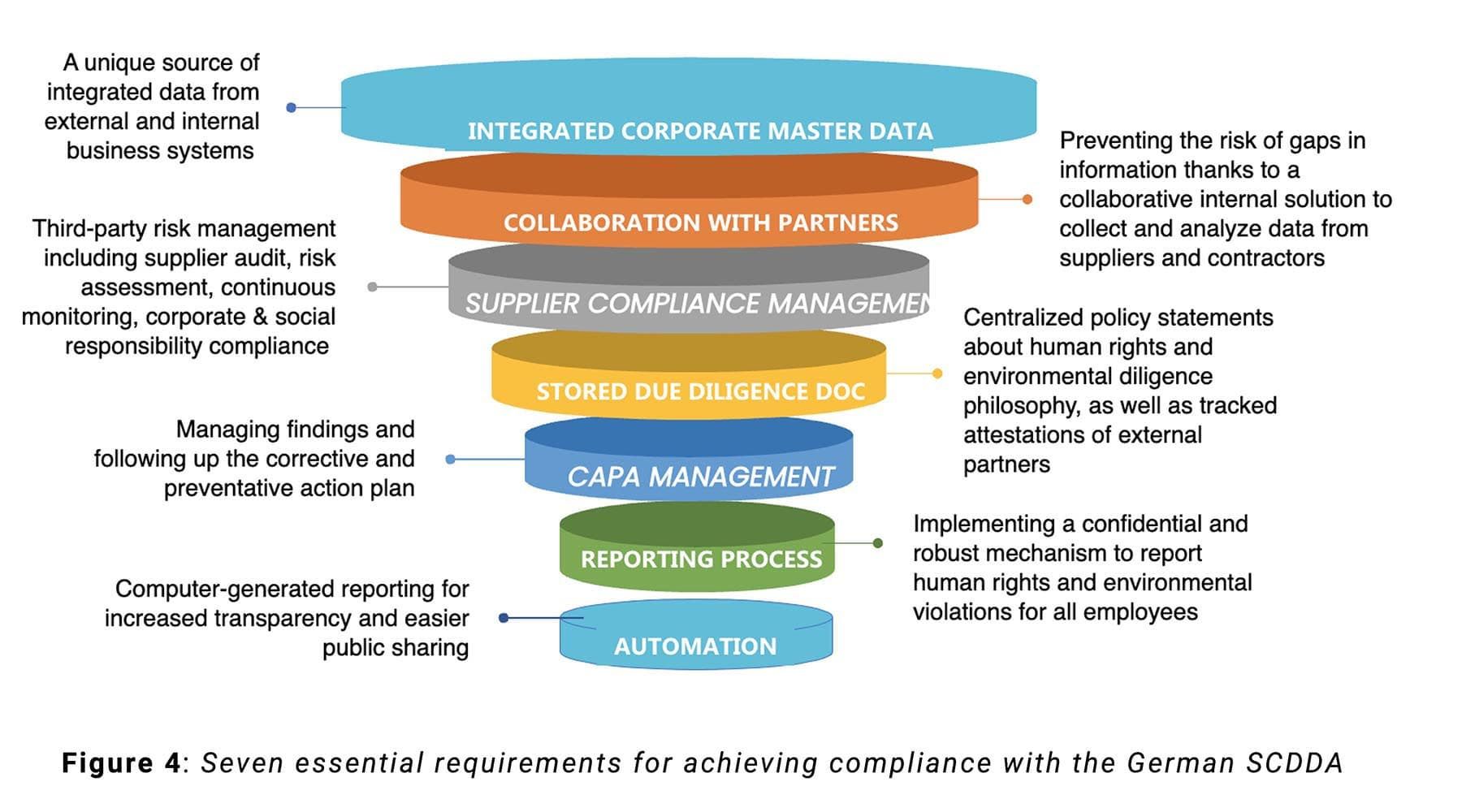
The new Act drives the implementation of an appropriate and effective risk management system that is integrated into all relevant business processes and captures all the demands and steps of this complex and resource-intensive process.
Enterprises must first perform a suitable risk analysis and define the responsibilities within their organization. Should any risk or abuse be identified, appropriate preventive or corrective measures must be taken to prevent or minimize violations. These include, for example, agreeing on adequate contractual human rights clauses with the direct/indirect supplier, implementing suitable procurement strategies, providing training, or taking supervisory measures.
Enterprises subject to the German Act are obligated to issue a policy statement which must identify the environment-related and human rights-related risks and any associated preventive and remedial measures.
The fulfilment of the due diligence obligations must be continuously documented within the companies, which must provide an annual report providing transparent information to the “Federal Office for Economic Affairs and Export Control” (“Bundesamt für Wirtschaft und Ausfuhrkontrolle”, BAFA). This annual report must be made publicly available on the enterprise’s website no later than 4 months after the end of the fiscal year and must be kept available there for 7 years.
In this context, the Federal Government has collected various forms of implementation support, which provide companies with the necessary know-how and offer assistance with concrete challenges: sector dialogues, advice & training services with guidance documentation.

Consequences on the Pharma Supply Chain
1. Compliance Challenges
A transparent supply chain, independent from the sector, plays a crucial role in improving environmental and social footprints and ensuring compliance with the German Supply Chain Act. In parallel, collaboration with direct and indirect suppliers is essential for ensuring that appropriate due diligence measures are taken and documented throughout the entire supply and value chain.
In the pharma sector, many international companies have been (proactively) improving their governance and compliance management capacities and investing in technologies that easily and automatically collect data on suppliers’ environmental and social practices.
2. Integrity Management
Integrity management, whether implemented internally or outsourced to an external agency, is an emerging area that supports corporations on how to apply:
-
- the highest ethical standards of product quality
- open and transparent communication and transparency
in all operational functions of their business as well as relationships.
In recent years, pharma companies have developed a great interest, and a stronger sense of responsibility, to always act with integrity. This field primarily arose in response to the increased concern from the general public about doing business ethically, which called on governments to legislate.
As a result, leaders need to innovate and ensure that high ethical standards are put at the heart of their business strategy. To complement the existing regulations and to present an entirely ethical view of the organization from within, pharmaceutical companies adopt different models, e.g., the Balanced Scorecard, Lean Six Sigma, and the Triple Bottom Line (“people, planet, prosperity”).
The pharma industry’s very purpose is to deliver medicines to improve patients’ lives (“People“). The management of manufacturing facilities is highly committed to environmental compliance and constantly endeavors to reduce waste and optimize productivity (“Planet“). Nonetheless, fulfilling their purpose as a business to produce drugs also results in significant profit (“Prosperity“), which overshadows their commitment to the first 2 “P” s, despite the pharma industry’s continual reinvestment of its profits into R&D to keep their pipelines full so their impact remains sustainable.
3. Reputational Impact
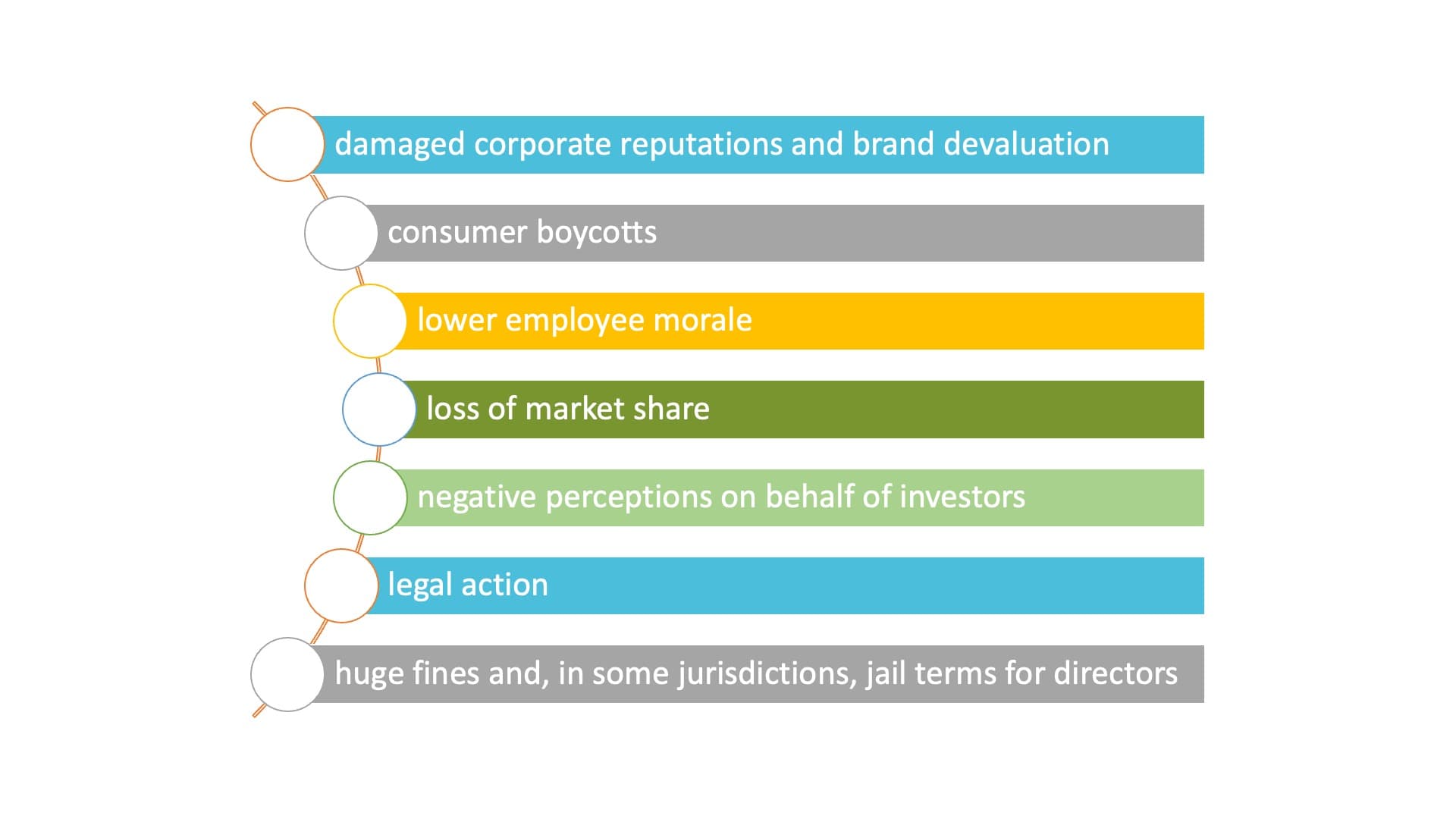
Companies are increasingly being held responsible for unethical behavior, including corruption, labor issues, poor transparency and accountability, inadequate corporate integrity systems and poor working conditions in their own operations, in those of their subsidiaries, as well as for the actions of subcontractors acting on their behalf (API (Active Pharmaceutical Ingredient) and packaging suppliers…).
The media has now made ethical transgressions easily public so that the consequences of such negative exposure can include:
Based on this observation, the pharmaceutical industry realized that early adopters of strong corporate ethics would thrive. Indeed, many pharma companies have embraced the social and environmental agenda in recent years. Consumers, patients and shareholders are increasingly ready to recognize the value of interrelated corporate responsibility and sustainability in international business to please the growing public’s demand.
4. Business Considerations
Some aspects for the pharmaceutical companies to take into account as regards to their respective management systems due to the explosion of legal requirements worldwide regarding social and environmental obligations:
-
- The financial market and business customers/patients are also increasingly demanding evidence of compliance with the relevant standards.
- Many international companies have been partnering with EcoVadis, the world’s most trusted provider of business sustainability ratings, by requiring their suppliers to conduct an assessment and/or an audit to evaluate their sustainability performance and work on improvements.
- Transparency requirements specific to large enterprises are also relevant for medium-sized companies since large enterprises demand proof of sustainability performance from their direct suppliers, also along their supply chains.
- Companies that meet these requirements particularly well increase their attractiveness as suppliers, while those who do not show any improvement run the risk of persistent criticism and decreasing/disappearing orders.
- Managing relevant sustainability topics along the supply chain is essential for future competitiveness and is considered an expression of a modern approach to business.
Conclusion
Germany will open the way as one of the first countries to advocate for social and sustainable business practices,and require corporations and suppliers to take responsibility for the consequences their corporate actions have on the world under sanction.
The pharmaceutical industry will be deeply impacted by this German SCDDA which opens a precedence in social and environmental practices. Therefore, all companies worldwide should consider implementing an to efficiently connect their data, processes, and people for supply chain transparency and compliance and be well-prepared for future requirements.
Companies worldwide are encouraged to take a voluntary and proactive approach to ensure their activities promote behaving responsibly, with fairness, sustainability, and cultural sensitivity. However, these concepts are intimately aligned with the pharma sector’s fundamental values, and only a few industries are absolutely committed to enhancing the quality of life as it is. Ethical pharma companies deserve recognition for being pioneers in the concept of sustainability before it has become so trendy.
Sources
- Wikipedia
- A Checklist for Complying with the German Supply Chain Act · Riskonnect
- Lieferkettengesetz startet 2023: Unternehmen klagen über Bürokratie (wiwo.de)
- CSR – Supply Chain Act (csr-in-deutschland.de)
- https://www.csr-in-deutschland.de/
- https://ecovadis.com/
- https://www.kroll.com/en/insights/publications/supply-chain-due-diligence-what-why-how
- https://accountability-framework.org/the-framework/topics/supply-chain-due-diligence/