IEC 60601: Safety and effectiveness of medical electrical equipment.
This standard specifies general requirements for basic safety and essential performance.
Collateral standards apply the 60601-1-3 standard states for radiation protection in diagnostic X-ray equipment. The other standards are related to the specific characteristic of all medical electrical equipment not fully addressed in the standard 60601-1, as standard 60601-1-2 states for EMC and 60601-1-8 for Alarms.
An electrical medical device needs to comply with the IEC 60601-1. Every point of the standard needs to be verified and referenced in a Compliance or Conformity Matrix. Tests must be conducted on the device to verify the compliance with the standard. Tests reports from certified ISO 17025 test lab have to be presented to the Notified Body as part of the technical file (technical documentation).
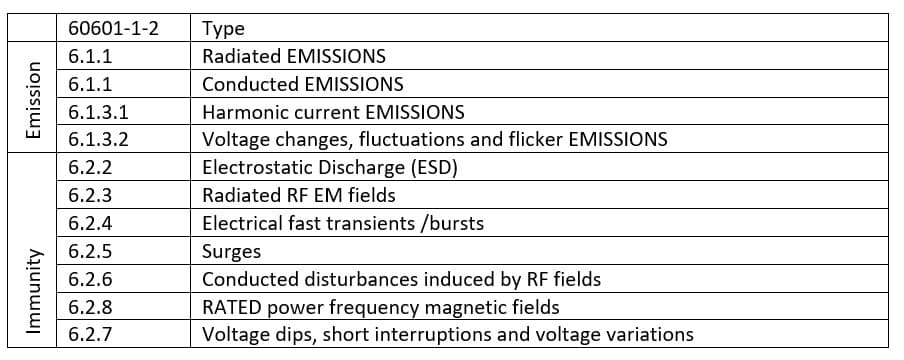
Electromagnetic Compatibility (EMC)
This electromagnetic compatibility is bidirectional. Indeed, the effect of electromagnetic perturbation generated by the product (emission) must be verified as well as the effect of the electromagnetic perturbation generated by surrounding equipment (immunity).
The maximum level of emission is defined in the standard. However, the acceptance criteria of the immunity of the device (the maximum level of surrounding electromagnetic perturbation supported by the device) depend on a risk analysis.
Different tests are conducted to prove that the emission of the device and its immunity conform to regulation.
Each test has its own standard:
60601-1 Protection against electric shock
Three differents classes exist.
The first one considers that the device is earthed to protect users from a shock. For this class of device, only basic insulation is required.
The second class of device is not connected directly to earth, so an increased effective protection against shock is required, to provide double insulation.
The last class of electric device is the internally powered device.
For all these devices, applied parts and accessible parts shall be specified in the instruction for use.
A safe medical product is not a product without any risks because absolute safety does not exist; only acceptable risks exist. Safety is defined in EN 14971 as “freedom from unacceptable risk”.
Author: Alix Auter, Life Science consultant KVALITO
KVALITO is a strategic partner and global quality and compliance services and network for regulated industries. To learn more about our service please visit us at www.kvalito.ch. If you would like to benefit from KVALITO’s expert services, feel free to send us an email to contact@kvalito.ch. Are you looking for an exciting and challenging position as a consultant? Please send your complete application to recruiting@kvalito.ch .





