This business case describes a project that KVALITO delivered for a Laboratory Information ‘Digital Solution’, which involved the implementation of a paperless GxP Process.
Project Scope
Implementation of a Laboratory Information Management System (LIMS) in Quality Control (QC) laboratory for a vaccine-producing pharma company on the following basis:
- QC test method protocols were transferred from a paper-based documentation system to LIMS.
- Implementation of LIMS interface to laboratory devices (i.e., balance, pH-Meter).
- SAP implementation in production areas and QC labs as leading documentation system for final test results, usage decisions and batch release.
- Implementation of LIMS-SAP interface for automatic transfer of GxP data from LIMS to SAP.
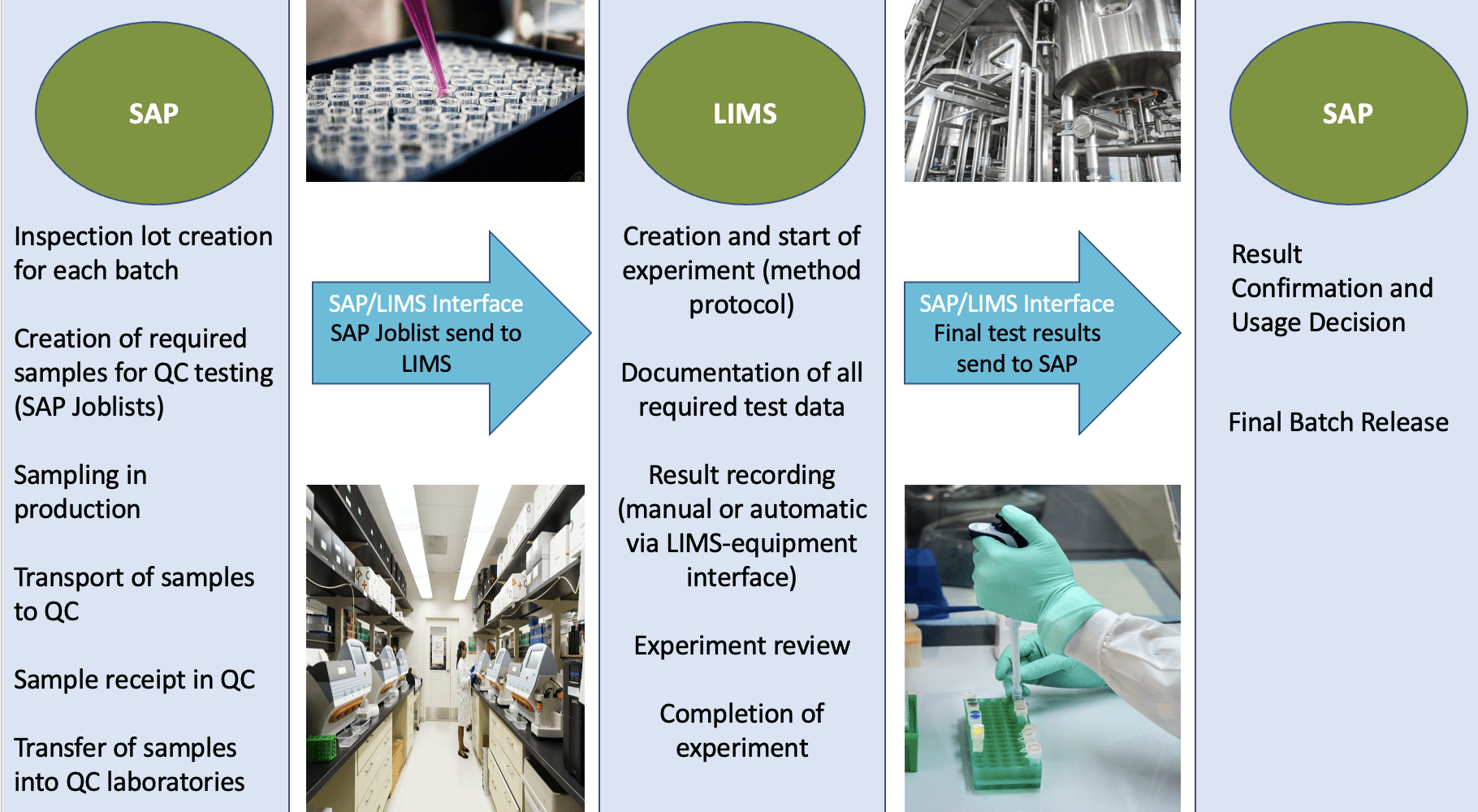
Figure 1 describes the process and data flow via production and quality control to final batch release after LIMS and SAP implementation: Inspection Lot is created in SAP for each batch, samples are taken in the production area (SAP), samples are transferred to QC (SAP) and tested in the QC laboratories (LIMS), QC test result data are transferred from LIMS to SAP, usage decision and final release for each batch are taken in SAP.
Figure 1 QC Paperless: Data Flow from production via Quality Control to final batch release, including SAP/LIMS Interface. Copyright KVALITO Consulting Group 2022
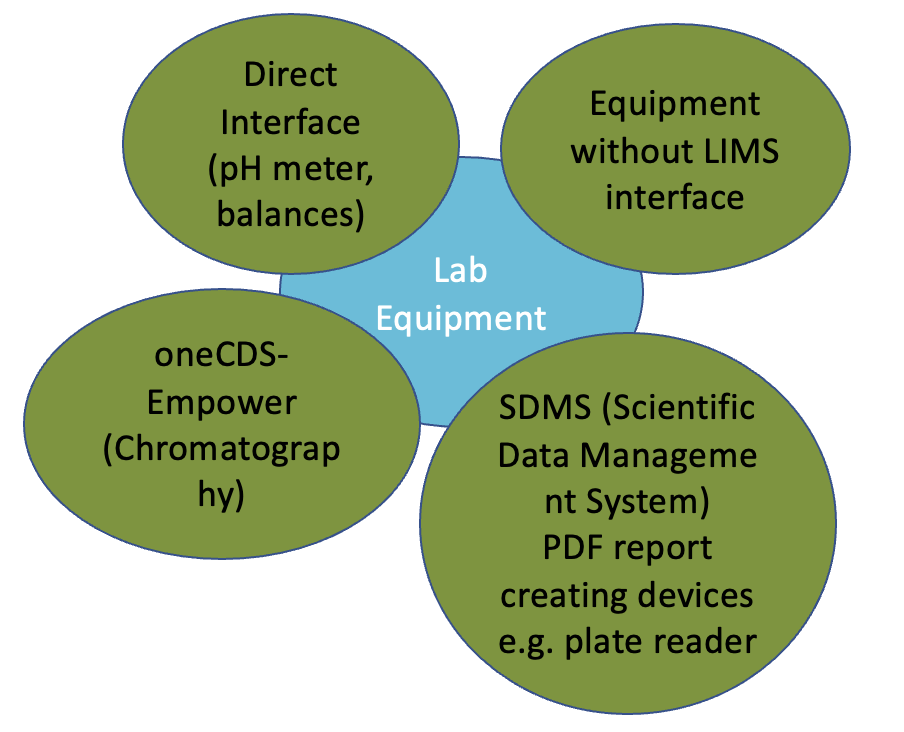
Figure 2 QC Paperless: Automatic data transfer from laboratory equipment into LIMS via direct SDMS or Empower Interface. Manual data transfer for laboratory equipment without LIMS interface. Copyright KVALITO Consulting Group 2022.
Challenges
This multisite Global project implementation raised specific challenges, for example;
- Global vs local stakeholder: Project led by international, whilst implementation on different sites with different requirements. Sites differ in systems/software/processes. This was well-managed and backed up by consistent, clear communication and discussion of local needs with global project stakeholders for an effective alignment process.
- Project Planning, including Project Scope and requirements, needed to be fully defined at the outset of the project. Roll-out vs development project: initially planned as LIMS roll-out project; however, each QC method was developed in LIMS based on global templates by local Admins and Lab Experts. This led to project delays because the initial project scoping needed to be completed/incorrect with the new requirements. Detailed initial project planning is essential, as well as setting up an effective change strategy that considers all stakeholders and project requirements.
- Communication and change management: resistance to the project in the lab and production area and doubting the “new system” can lead to a lack of project support, so transparent communication is instrumental in getting all stakeholders working towards the same goal and understanding the project as well as its future benefits.
- Knowledge & Training: LIMS knowledge and experience in training activities while the project was already running can lead to delays. Therefore, delivering comprehensive training in advance of the commencement of the project for critical users is imperative. LIMS knowledge and training are essential for admins and lab experts to create the methods in the new system. Establishing a knowledge-sharing process from system experts/critical users in the lab/production space is crucial.
- Technical/IT challenges: data transfer regarding LIMS/SAP interface and equipment/LIMS interface. Complete testing processes for all interfaces and data flows: SAP to LIMS, Equipment to LIMS, and LIMS to SAP must be in place to avoid deviations in the routine business and to assure compliance with data integrity requirements.
- Systems (LIMS/SAP/Empower/SDMS) and required interfaces provided, set up and validated by the global project team must always include local stakeholders in validation processes to build up and guarantee a robust system, process and interface knowledge.
What KVALITO did
Quality Assurance
- Business Change Control Strategy and Management
- SOP Review and Approval
- Approval of QC methods and equipment interface (direct, oneCDS (Empower), SDMS) in the new laboratory management system: testing of each method protocol in the new LIMS test environment, documentation of testing in specific checklists, approval of checklists and method incidents in LIMS by QA, Admin, Business
- Consultation for questions and issues related to compliance topics (GMP, GLP, CSV, Data Integrity, Pharmacopoeia)
- Deviations, CAPA Management
- Organisation and planning, problem-solving, and analysis of business processes for optimisation
- Additional project support: translation of training material, execution of the training sessions
- Audit preparation & readiness
Project Management
Project Management, Planning & Optimization of Business Processes:
- Management of local workstreams: LIMS Admins, SAP Master Data, Business Change Control & QA, Training, Change Management & Communication, IT Lab Support, Lab Key User
- Milestone and Resource Planning, Kick-Off Meeting
- Using RACI Matrix to clarify roles and responsibilities in the project
- Defining timelines according to different steps in the method implementation process Method Implementation: weekly planning for status updates & direct blocking point detection in the method implementation process (Method Role Out Plan in Excel, updated twice a week in team meetings)
- Visualising the detailed method implementation process flow and local/global roles involved (Swimlane Diagram): process understanding and detection of blocking points in the process for improvement
- Transparent & clear communication: meeting minutes to avoid misunderstanding, align on actions and their owners and allow efficient follow-ups
- Business Cut Over Plan for Go-Live Planning: defining dependencies, actions and responsibilities between workstreams (tracking in Excel for Action Item, Due Date, Action Owner, Status)
- Hypercare Organization after Go-Live: Teams Chat, Shared OneNote, Tracking of Issues and Status
- Regular team meetings: daily, weekly, report meetings to global stakeholders
- The organisation of workshops for blocking point detection, problem-solving, decision-making, communication purposes, training
- Project presentations, newsletter, and workplace posts for transparent communication about project status and timelines
- Stakeholder Management: Coordinated activities with multiple stakeholders: global project leads, local project lead, QC Head, QC Labs, QA, IT Lab Support, QC Applications, System Owners (SAP/LIMS)
People, Processes and Tools
People / Roles (local)
- Project Management
- Quality Assurance
Tools and Technologies:
- LIMS SAP QM
- Office 365
- MS Project Management
- Trackwise
- Documentum
- Veeva Vault Quality
- Microsoft Teams
- Scientific Data Management System (SDMS)
- OneCDS (Empower)
Processes:
- Full Life Cycle Project Management following PMP, Agile PM, Scrum
- Strategic Planning and Analysis
- Quality & Performance Optimization
- Full Quality Assurance Support
- GxP and Compliance Processes (FDA, PICs, cGMP, Pharmacopoeia)
- Team and Time Management
- Issue Identification and Resolution
Value Delivered
- Knowledge of regulations for pharma production and quality control (FDA, GMP, GLP, CSV, Data Integrity, Pharmacopoeia)
- QA competencies (Review/Approval, Change Control Risk Assessment (FMEA))
- Team working skills (QA-Business, Project-Business)
- Building a client-consultant relationship based on transparency and trust
- Open communication skills
- Project management and organization skills (Agile, Scrum, RACI, Swimlane, Meeting Minutes)
- Hands-on mindset
- Successful project delivery: meeting project goals/milestones on time
- Knowledge of technologies and software skills concerning digitalization and future lab
Clients/References
- GSK