The pandemic lockdown has sped up digital transformation in this era. It is increasingly accepted that the...


The pandemic lockdown has sped up digital transformation in this era. It is increasingly accepted that the...
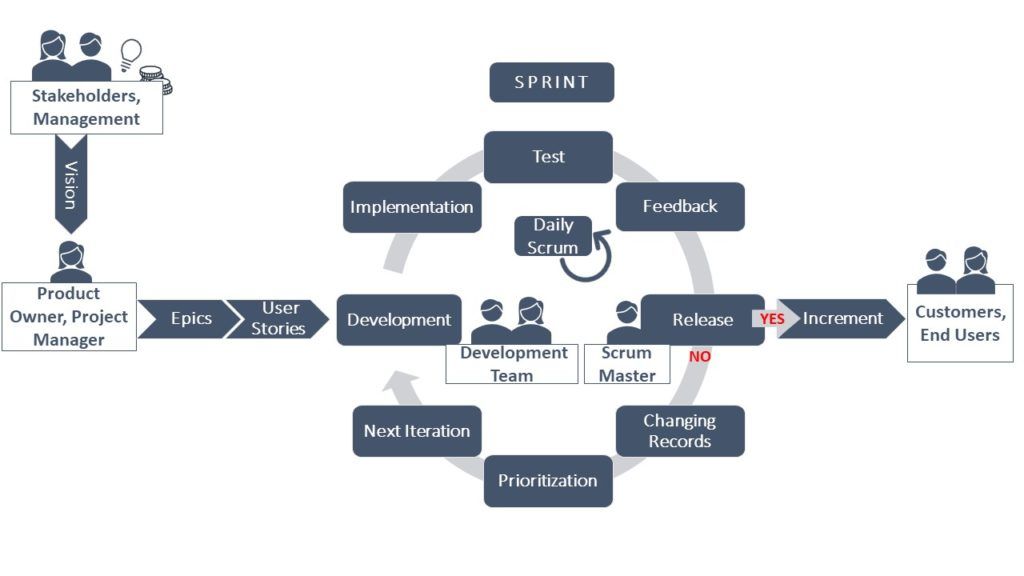
“Intelligence is the ability to adapt to change.” (Stephen Hawking) Introduction To date, classical methodologies,...

Precision Medicine Precision medicine, also known as personalized medicine, is an approach to developing safer novel...
Two of our consultants recently represented KVALITO at the 15th DIA Conference on European Electronic Document...
“I think the biggest innovation of the 21st century will be at the intersection of biology and technology. A new era...
If you are in the pharmaceutical or in the medical devices industry and cannot sleep at night that may very well be...
What we understand today as “social media” has been with us for about a decade now. And the use of Facebook, Twitter,...