Classification of software as a Medical device under Medical Device Regulation (European Union)
The first step is to determine whether your software is a Medical Device.
How do you determine if the software is a Medical Device?
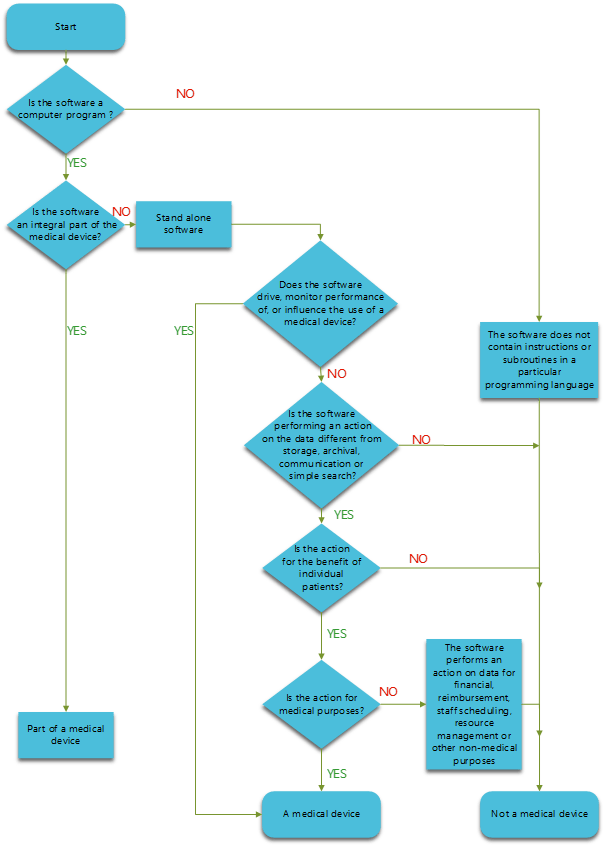
Figure 1: How to determine if the Software is a Medical Device. Adapted from COCIR Contribution, Decision diagram for the qualification of Software as Medical Device. Copyright 2020, Kvalito Consulting Group.
If your Software is a Medical Device or a part of a Medical Device, it has to be classified.
How to classify a Software as a Medical Device under MDR?

Figure 2: How to classify a software as a Medical Device. Adapted from the Medical Device Regulation of the European Union, Copyright 2020, KVALITO Consulting Group.
Rule 11 refers to software as a Medical Device:
“Software intended to provide information which is used to make decisions with diagnosis or therapeutic purposes is classified as class IIa, except if such decisions have an impact that may cause:
- death or an irreversible deterioration of a person’s state of health, in which case it is in class III; or
- a serious deterioration of a person’s state of health or a surgical intervention, in which case it is classified as class IIb.
Software intended to monitor physiological processes is classified as class IIa, except if it is intended for monitoring of vital physiological parameters, where the nature of variations of those parameters is such that it could result in immediate danger to the patient, in which case it is classified as class IIb. All other software is classified as class I.”
Author: Alix Auter, Life Science Consultant, KVALITO
KVALITO is a strategic partner and global quality and compliance services and network for regulated industries. To learn more about our service please visit us on www.kvalito.ch
If you would like to benefit from KVALITO’s expert services, feel free to send us an email to contact@kvalito.ch.
Are you looking for an exciting and challenging position as a consultant in Europe? Send your complete application to recruiting@kvalito.ch.




