Development and Validation of Digital Pathology
Digital pathology means the use of technology to accelerate and improve the workflow in pathology laboratories. It focused on data management based on information generated from digitized specimen slides by using virtual microscopy. This virtual microscopy uses digital scanners to capture digital slides, store them, analyze them and finally, share them with the scientist. These scanners mimic the conventional microscope with additional software that visualizes and analyzes the captured images.

Ultimately, artificial intelligence algorithms can be applied to automatize or support the pathologist diagnosis. It represents a significant potential to detect anomalies and forecast disease trajectories. As the slides are gathered and stored, it creates a bank of data which can be used to train machine learning models and increase their predictive accuracy. The clinicians will get insights into disease advancement and their potential outcomes.
Nevertheless, AI models needs to be transparent, unbiased and ethical to be used by in healthcare systems. According to a study published in JAMA Network Open, 63% of the surveyed had concerns regarding the “black box” nature (without knowledge of its internal workings) of AI decisions.
The practice of digital pathology, also called Whole-Slide Imaging (WSI) saves time on tasks, such as inspecting glass slides and analyzing tissue samples directly from the slide. This saved time can be allocated for diagnostic purposes.
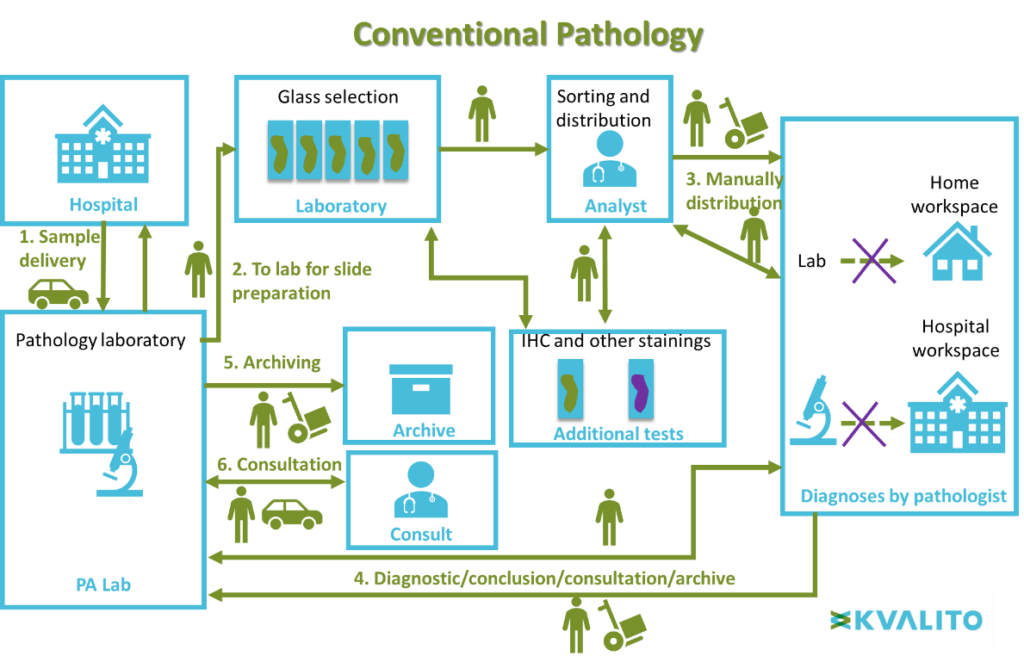
This layout illustrates the difference between conventional and digital pathology.
Digital Pathology, June 2019.
Conventional microscopy workflow:
First, the biological sample is delivered from the hospVital to the pathology laboratory. Slides are then prepared with the biologic sample in the laboratory. The analyst sorts the slides manually and distributes them to a pathologist for diagnosing. Then for the conclusion, diagnostic results are sent back to the pathology laboratory for archiving and the information can be used in a medical consultation.
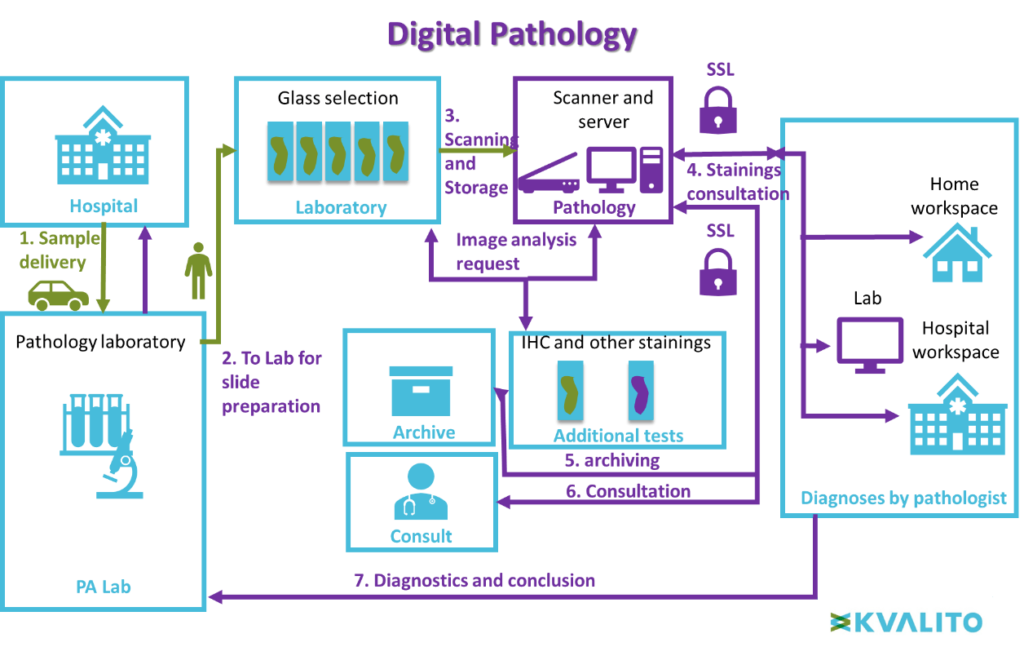
Virtual microscopy workflow:
Contrary to conventional microscopy, after preparation, slides are directly scanned and stored in a secured server. Pathologists can consult the scan of the slide directly online (from every place within a secured server). The result of the diagnosis is sent to the pathology laboratory. No manual logistic is necessary.
Digital pathology allows for a faster and more efficient workflow by sharing images captured around the world. It also has better outcomes for patients, allows the pathologist save time, and s/he can provide a fast and accurate diagnosis.
Digital Pathology Benefits:
We can see which evident benefits are coming together with this new approach:
- As physical transportation is not necessary, workflow is optimized, allowing for fast diagnosis. The images can be shared with teams spread around the world in a matter of minutes. Access to the sample image is greatly enhanced as the need to go directly to the lab to put the slide on the microscope falls away. The pathologist could also perform his diagnosis from home.
- Huge saving in costs for the hospital as the need for numerous physical transportations and logistics, on which billionaire companies have focused their business, is eliminated.
- The availability of many of these data (images) at once in a matter of minutes instead of days, connecting several teams spread around the world. This can help to obtain a consultation from someone specialized in a specific area who is located far from the lab where the sample has been taken.
- New insight from analyzing a large dataset
Digital Pathology Disadvantages
Together with the above-quoted benefits, we also need to mention what, at this time, is still to be considered a flaw moving through this new approach:
- with glass slides, the pathologist can change the magnification in real-time. With digital data, the magnification is pre-setup by the istotechnician, and then it is fixed when it arrives at the pathologist.
- Massive one-time money investment to set up the infrastructure. None the less, it will result in a considerable saving of resources in the long run.
- Blurring: tissues sample are not always even. It can have blurred areas because different thickness may require different magnification on the same sample. As the magnification is pre-set and equal for the whole sample, some parts could result in given areas that are out of focus.
- Missing or fading parts of the tissue: The scanner does not always capture a full-colour intensity, and the actual colour may not display properly on the digital image taken by the scanner.
Most of these contras are a current technological limitation which can undoubtedly be improved if not eliminated, with more investment in research.
Some diagnostic equivalence studies have been performed, comparing the diagnosis outcome made from the same pathologist, assessing the physical glass the first time, and the related images after two months. So far, diagnostic equivalence is estimated to be 95%. The 5% which was not entirely equivalent has no clinical effect.
Quality and regulatory requirements for marketing digital pathology device
US requirements:
In the USA, digital pathology implementation needs to follow the Clinical Laboratory Improvement Amendments (CLIA) and the College of American Pathologist (CAP) Regulations.
The manufacturer needs to apply a Premarket Submission (510(k)) for software contained in a medical device. This device is an In-vitro diagnostic, classified as a Class II device, with special controls. The review of the premarket submission will be done by the office of in-vitro diagnostics and Radiological Health (OIR) .
In general, the documentation provided in the submission should (based on FDA Guidance for the Content of Premarket Submissions for Software Contained in Medical Devices) :
- Describe the design of your device.
- Document how your design was implemented.
- Demonstrate how the device produced by your design implementation was tested
- Show that you identified hazards appropriately and managed risks effectively (including cybersecurity)
- Provide traceability to link together design, implementation, testing, and risk management.
EU requirements:
Virtual microscopy is used for human or veterinary pathology. It has a wide range of applications such as drug discovery, disease diagnosis, teleconsultation, training and education of pathologist.
In the EU, digital pathology is following the new In-vitro diagnostic regulation. It needs a CE marking and approval of the conformity assessment by a notified body. The classification depends on the intended use. This kind of device is composed of an electronic device, the scanner, and software. It helps the pathologist to diagnoses. According to its clinical use (e.g., screening, diagnosis, or staging of cancer), a WSI slide scanner can be classified as a Class C device. So a performance evaluation, including a scientific validity report, an analytical performance report, and a clinical performance report is needed. A special regulation is applied to companion diagnostics.
QMS needs:
To implement digital pathology system, clinical laboratories are required to implement organized and documented archival, retrieval and logistical procedures. Especially a process describing the handling of original slides/blocks for consultation and legal procedures. The archival, retrieval and logistics of pictures is managed by the Pathology Picture Archive and Communications Systems (PACS). Tests reports can be stored in pathology PACS, and they are subject to the same regulatory requirements as those created and stored in a laboratory information system (LIS).
IT Infrastructure Requirements
As every image taken from the slice scanned is between 500 Mb and 2/3 Gb (depending on magnification) massive virtual storage is required instead of a vast physical archive where to store the glass slides.
Such virtual archives have also to be high-performance to upload, transfer and recall such heavy files in a reasonable amount of time.
Such files also contain critical and personal patient data so that the connection used to upload and transfer data should be a secure one (SSL), and data should be encrypted. The archive itself needs to comply with Data Privacy and Data Security regulations.
The hospital or lab where the tissue sample is taken need also to be provided with scanners of several dimensions as the slices can spread from 1×3 up to 6×8 (for brain sections) inches.
Digital pathology labs also need to have complex image viewer software that does not belong to the scanner itself.
Ultimately, artificial intelligence algorithms can be applied to automatize or support the pathologist diagnosis.
Validation Approach:
Validation of a digital pathology system (DPS) is necessary for clinical use to ensure laboratory compliance to State regulations, to protect patient safety and confidentiality, to assure digital pathology data is accurate, and to maximize the value of a DPS.
Validating a DPS is also useful to create a baseline for acceptable performance while using a slide imaging scanner, data management software and image analysis systems in a laboratory environment.
Characteristics of a strong foundation for a DPS validation include:
- An anatomic pathology specimen tracking system (i.e. barcodes) from access, through sign-out, and archival of blocks and slides. Every slide has a unique ID.
- Experience qualifying digital imaging devices for clinical use (i.e. Cytogenetics, FISH systems) that may or may not be FDA approved
- Procedures for data retrieval and preservation of whole slide images
- The laboratory applies all current American Society of Clinical Oncology (ASCO) & College of American Pathologists (CAP) recommendations and current CAP checklist guidelines to all methods used to create stained slides and has conducted the appropriate verification and/or validation required.
Although the CAP draft guidelines do not require validation of each component of a DPS, this white paper will discuss many of these individual components and discuss what areas should be reviewed and monitored throughout the validation process. According to CAP, validation should encompass the entire Whole Slide Imaging (WSI) system. Such a process should include a sample of at least 60 cases (patients) for one application. Validation study should non the less establish diagnostic concordance between digital and glass slides for the same observer. A washout period of two weeks should occur between viewing glass and digital slides. Concordance should be no less than 94-95% according to such recommendations.
Hardware and software validation and qualification shall follow the common GAMP5 validation approach, driven by the regulation applicable to the specific item.
As hardware, we include the scanner which is considered because of its purpose, as medical device and computer.
As software, we consider the viewer software, which can be part of the scanner itself being part of the same validation or run independently, requiring its validation as Software as Medical Device (SaMD). Another essential software to be considered is the Data Management tool due to the vast amount of data as each slide can have a size that spread from 500 Mb to 2 – 3 Gb.
Also, AI algorithms, which are becoming more and more integrated within the new digital solutions, would require validation. A validation process starts by determining the scope of the model as well as how it relates to other algorithms. After these initial steps, the validator analyzes various aspects of how model input data is managed and continues by measuring model performance.
Agile Project Management to Implement Digital Pathology Project
With many project management methodologies to choose from, being Agile, Waterfall, Kanban or Lean, each one with their own set of rules, principles, process and practices, adopting the “right” methodology will depend entirely on the type of implementation and also the company itself.
Every implementation varies in scope and requirements, which means the “right” methodology to use will also depend on the different project specifications.
Most companies use waterfall to handle one or more phases — such as planning — when the fulfilment of previous requirements is necessary in order to be able to proceed with next activities, and where these do not require rapid or repetitive steps. Complex projects require specific and detailed planning, in particular, large implementations require a more comprehensive, systematic, often slower approach to defining, analyzing, and documenting every aspect of a project, making waterfall a better approach.
Once a project enters the development phase, rapid and repetitive changes require a different approach, and this is where agile kicks in to deliver the best results in the shortest amount of time.
Taking into account the specific nature of the development of a digital pathology implementation, following an agile approach to speed up distinct implementation phases is recommended, when it comes to software development or testing, and combine it with other methodologies like waterfall or lean to create a hybrid solution.
A hybrid approach aids in making agile even more adaptable within various industries or to suit the unique nature of a project, product, or service. Again, due diligence is required to determine the suitability and capacity of the different methods and processes available.
Authors: Alix Auter LIfe Science Consultant KVALITO, Marco Polisena Life Science Consultant KVALITO
Clara Sayrol Life Science Consultant KVALITO
KVALITO is a strategic partner and global quality and compliance services and network for regulated industries.
If you would like to benefit from KVALITO’s expert services, please send us an email to client.partner@kvalito.ch.
Are you looking for an exciting and challenging position as a consultant? Please send your complete application to recruiting@kvalito.ch




