Transformation- Cell & Gene Therapy Platform Operations & Compliance
Design, development and implementation of complex orchestration platforms enabling (personalized) Cell & Gene Therapies (CGT) and maintenance of compliance through operational services, change and deviation management.
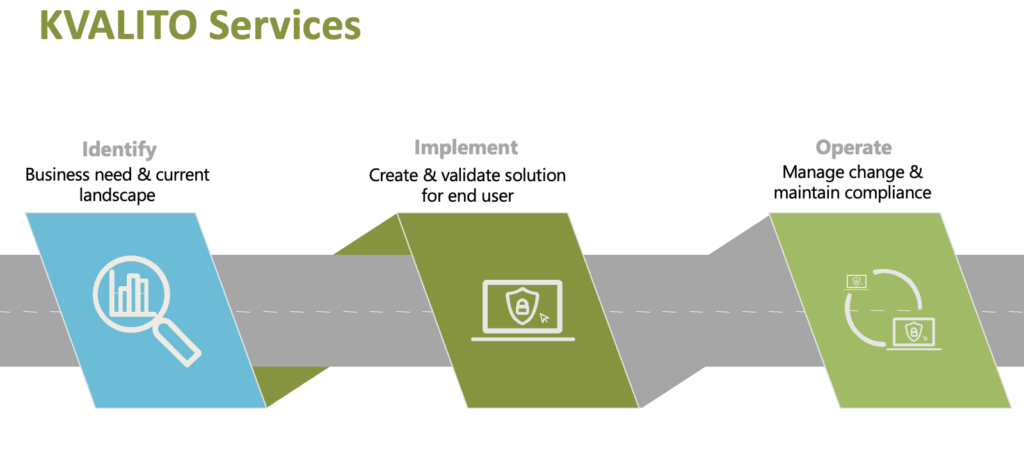
Challenge
Orchestration platforms enabling (personalized) Cell & Gene Therapies are highly complex, as integrated computer systems enable a Therapy by maintaining the Chain of Identity of the product throughout the End-to-End Supply Chain.
The supply chain processes to support personalized CGT are very complex and differ dramatically from those of standard pharmaceutical products and the regular channels through which these products are distributed. For CGT products, depending on the nature of the product itself, its logistic and storage requirements, as well as required turnaround times to patients, bring unprecedented challenges to those in charge of designing, implementing and operating CGT orchestration platforms.
These orchestration platforms are used by a great variety of external (nurses, doctors and pharmacists at hospitals) and internal (supply chain professionals, customer service, personnel at manufacturing plant, etc.), all of which carry out different activities in the system and have diverging needs and expectations. Creating one integrated system that enables all of these functions is very challenging. User requirements for these functions can vary significantly given the different tasks carried out by the respective functions. Hence, the creation of one product that takes into consideration all user requirements from the various functions is very challenging and requires a lot of due diligence, as the implementation of constantly changing business requirements and a great user experience do not always go hand-in-hand, which results in a balancing act.
As various functions use these CGT orchestration platforms in different locations, platform integration with existing IT-Infrastructure is a challenging aspect of the design and implementation. Integration with internal systems used by the client e.g. Manufacturing Enterprise Systems (MES), Customer Relationship Management (CRM) systems, Enterprise Resource Planning (ERP), as well as external systems owned by logistics providers, need to be considered and managed to customers expectations.
Requesting, managing and performing changes to these highly complex and highly integrated systems is a very challenging undertaking, as it involves a large group of stakeholders and requires a good granular understanding of the processes the CGT orchestration platform enables, as well as subject matter expertise in change and deviation management. Operational support requires substantial knowledge of the IT technology (SAP, Salesforce.com) and an in-depth understanding of the respective platform and the processes it supports. This comprehensive know-how is required to identify the origin of issues and deviations that occur and dissolve them for the customer.
What KVALITO did
- Provided consultants responsible to build and lead teams / work streams as part of the organizational governance driving the platform design and implementation
- Design of business processes and data flows, use cases and definition of user requirements
- Creating traceability of requirements, processes, as well as their documentation and training materials, to enable effective and efficient change management by utilizing TIBCO NIMBUS
- Execution of risk and data integrity assessments
- Creation of training material
- Project planning and management of platform releases
- Leading and enabling changes to the CGT orchestration platform by managing change requests throughout the entire lifecycle, incl. stakeholder alignment and approval in change board
- Leading deviation management activities, incl. ownership and investigation of deviations and quality events, and definition of corrective and preventive actions
- Admin function to support operational activities and solve urgent issues for the end-user
- Business relationship management
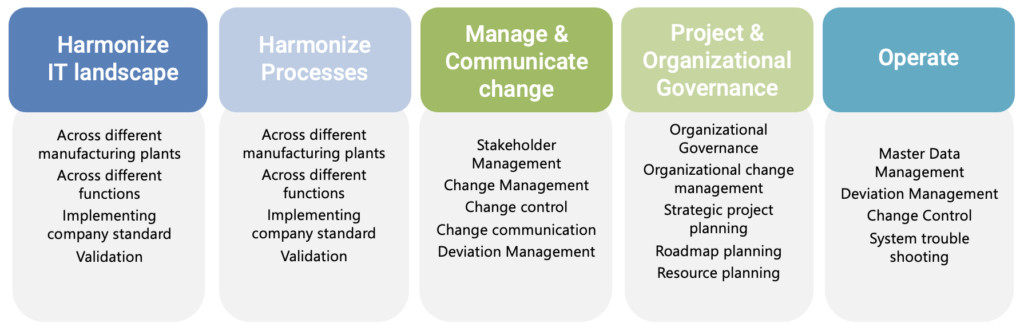
People, Processes and Tools
People / Roles
- Business Process Excellence
- Governance & Organizational Change Management Lead
- Change Control Excellence
- Deviation Management Excellence
- Master Data Manager
- System Admin
- Change Management Lead
- Change and Communications Manager
- Enablement Lead
- IT Project Manager
- QA/eCompliance / Validation Subject Matter Expert / CSV QA
- (Project)Quality Manager
Tools and Technologies
- Salesforce.com
- SAP ECC
- SAP S4/HANA
- Dell Boomi
- Airtable
- Vineti
- TIBCO NIMBUS
- JIRA
- HPLM
- Trackwise
- Oracle
- Confluence,
- X-Ray
- MS Azure,
- HP ALM,
- HP ServiceDesk,
- HP Quality Center
- SAP Solution Manager
- Veeva Life Cycle Management
- SAP BI
- Tableau
- IBM Watson
- SNOW
Processes
- End-to-End supply chain process
- Operational support
- Global change control process
- Global deviation management process
Value Delivered
- Enabling personalized medicines by implementing a Chain of Identity application (individualized medicines, treatments, products)
- Enabling product launches globally
- Enabling Manufacturing site launches, incl. contract manufacturers (CMO)
- Putting the patient in the centre of the solution (individualized medicines, treatments, products)
- Trouble shooting on orders when issues occur in system admin function
- Enabling efficient change management by creating traceability of processes, requirements and relevant documentation.
- Improving Solution-Centered Approach (no onesize fits all)
- Increasing productivity by implementing system automations and redesigning processes
- Leveraging knowledge and resources
- Saving time and costs through system automation
- Increasing GxP compliance by owning change control and deviation management processes
- Improving business experience by improving UX design
Clients / References
- Novartis
- Genentech / Roche




