What is usability ?
The new Regulation, MDR, strengthens the rules regarding labelling and usability.
Usability is the characteristic of the user interface that facilitates use and establishes effectiveness, efficiency and user satisfaction in the intended use environment.
In parallel, usability engineering is defined as Human Factors Engineering. It is applying knowledge about human behavior, abilities, limitations, and other characteristics to the design of medical devices (including software), systems and tasks to achieve adequate usability.
Usability have an impact on safety and performance of MD. Indeed with the right usability, some medical errors, injuries can be avoid.
MDR :
“Reduce, as far as possible, the risks related to the ergonomic features of the device and the environment in which the device is intended to be used (design for patient safety)”
What is the current harmonized standard regarding usability?
IEC 62366
The latest standard is IEC 62366-1:2015 but the harmonized standard is IEC 62366:2007
So the latest version may not be used for the statement of compliance
Usability dimensions
- The intended use : the purpose for what the device has been designed
- The users : person operating or handling the medical device
- The use environment : the intended contest for using the device (expected worst conditions)
- The user interface : design define based on user requirements
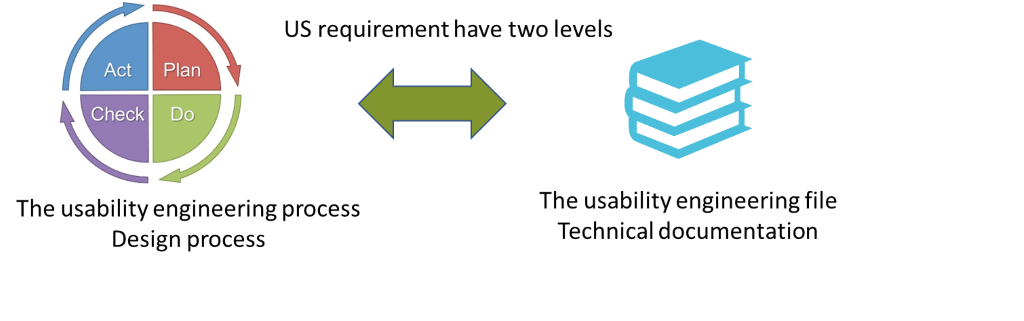
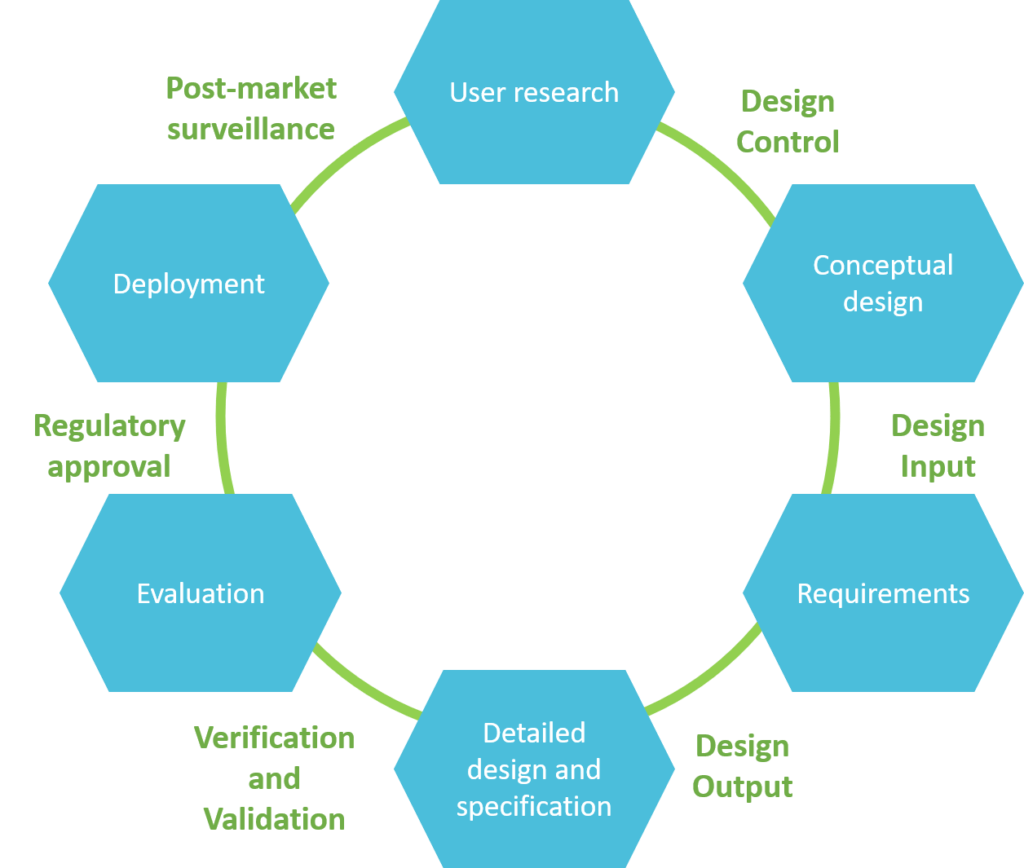
Usability engineering process
II. Labelling : Unique Device Identifier
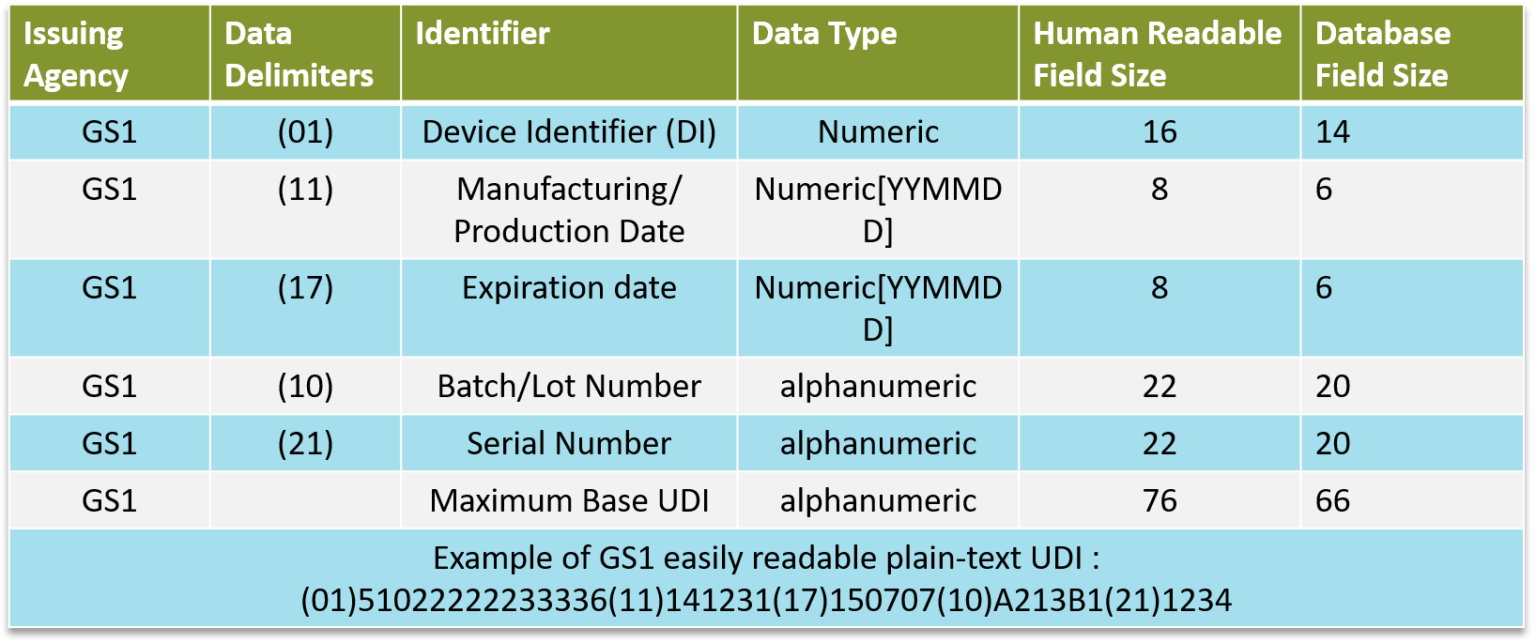
UDI = DI (Device Identifier) + PI (Production Identifier) + Database
DI : specific to a manufacturer and a device group
PI : specific to a particular device
Databases : Europe = EUDAMED, US = GUDID
3 accredited agencies for assignment of UDIs according to UDI rule:
GS1 : Global Trade Item Number + Application Identifier
HIBCC: Health Industry Business Communication Council
ICCBBA: International Council for Commonality in Blood Banking Automation
USA and EU : labelling = stickers + instruction for use

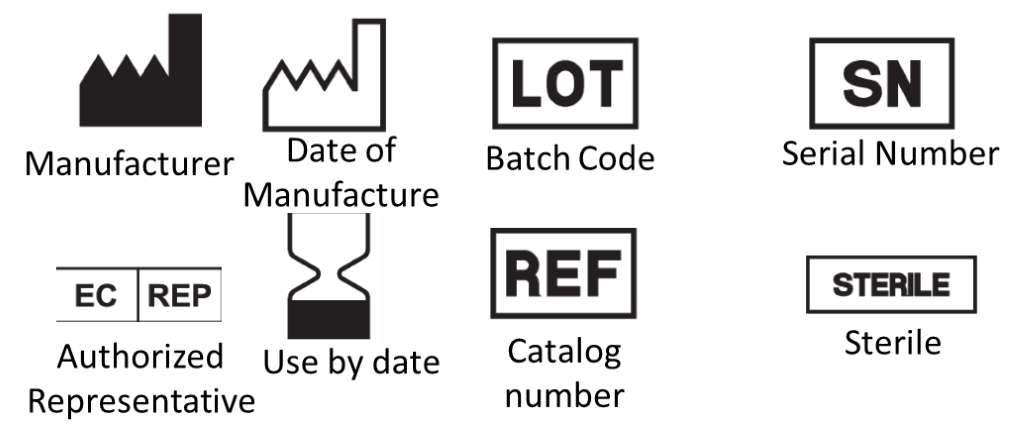
Purpose of labelling :
- identify the medical device and its manufacturer
- communicate safety and performance-related information to the user, professional or lay, or other people as appropriate
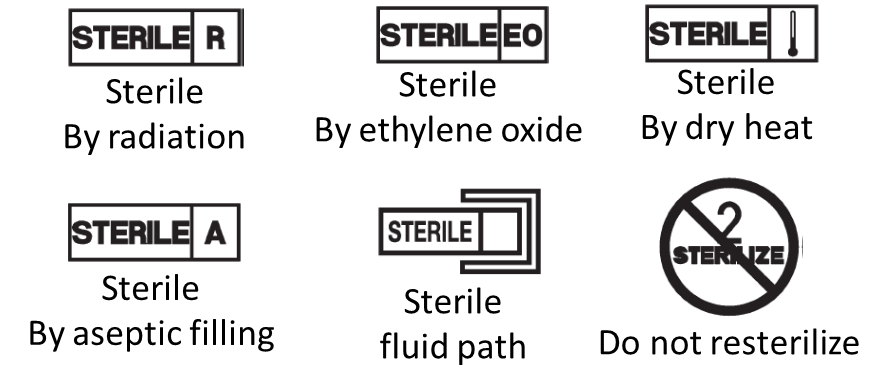
Prohibited to use text, names, trademarks, pictures and figurative or other signs that may mislead the user with the intended purpose of the device.
So labelling must be verified (meet all the requirements) and validated (check with a real user, including usability).
Author: Alix Auter Life Science Consultant, KVALITO
KVALITO is a strategic partner and global quality and compliance services and network for regulated industries. To learn more about our service, please visit us on www.kvalito.ch
If you would like to benefit from KVALITO’s expert services, feel free to send us an email at contact@kvalito.ch. Are you looking for an exciting and challenging position as a consultant in Europe? Send your complete application to recruiting@kvalito.ch.




